This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
The impact of peripheral artery disease (PAD) on patients' health status and the processes of PAD care have not been prospectively documented in patients with new or worsening claudication.
Objectives:
The PORTRAIT (Patient-centered Outcomes Related to TReatment Practices in Peripheral Artery Disease: Investigating Trajectories) study prospectively defined health status outcomes and then related aspects of patient care to these outcomes in patients with new-onset or worsening claudication. In phase I, we developed and tested the study's design. We are now reporting on phase II, in which we expanded the study to 10 US PAD specialty clinics. The following are our research questions: (Aim 1) Do PAD treatments differ by patient characteristics? (Aim 2) Is earlier, rather than later, revascularization associated with more rapid improvements in patients' health status? (Aim 3) Does treatment of PAD vary by physician and health system? (Aim 4) Are variations in care associated with differences in patients' outcomes? (Aim 5) We created educational materials to inform future PAD treatments.
Methods:
PORTRAIT enrolled 797 patients with new or worsening claudication symptoms from 10 US PAD specialty clinics. Before treatment, we interviewed patients and abstracted clinical services information from their medical records. We collected health status information at 3, 6, and 12 months follow-up.
Results:
PAD specialist referred about 1 in 5 patients for an invasive treatment but only less than 2% patients for supervised exercise therapy. Adherence to individual PAD performance measures was relatively high for antiplatelet therapy (84%) and statin use (90%). When we defined perfect care as meeting 4 quality measures of PAD care (statin therapy, antiplatelet therapy, smoking cessation, supervised exercise therapy), only 4.6% of patients received perfect care (aim 1). The magnitude of change in patients' PAD-specific health status scores was greater at 3, 6, and 12 months follow-up in patients who underwent invasive treatment vs those who did not receive invasive treatment (aim 2). Adherence to individual PAD performance measures varied considerably across enrolling PAD clinics (aim 3). Patients who had been receiving evidence-based care (a statin and antiplatelet therapy) had better health status scores at baseline and at all time points except for 12 months (Peripheral Artery Questionnaire summary scores), as compared with those who were not receiving these measures. Associations between perfect care (4 quality measures) and health status outcomes were less clear (aim 4). Women and minority-race patients had consistently lower baseline and follow-up health status scores (aim 5). We created the framework for patient educational materials in collaboration with patient advisors.
Conclusions:
While prescription rates for the medical management of the cardiovascular risk in PAD were relatively high, supervised exercise therapy—a performance measure—was seldom prescribed. Women and minority-race patients were at risk of experiencing a lower health status level. Receiving better quality of care following PAD evaluation at baseline was associated with better health status levels at 3-month and 6-month follow-up. Educational materials will be used to share insights learned from PORTRAIT.
Limitations:
Findings can be generalized only to the specialist care setting; in an observational study, the potential for residual confounding and selection bias mandates cautious interpretation; further cross-validation of risk estimates derived from health status prediction models in PORTRAIT is needed.
Background
About 8.5 million Americans aged 40 years or older are living with peripheral artery disease (PAD).1 In PAD, the underlying pathophysiologic process, atherosclerosis, presents itself as blockages in patients' leg arteries that prevent adequate blood flow, which can result in burning calf (or thigh or buttock) pain while walking and that is relieved with rest (intermittent claudication). While the onset of PAD tends not to be as abrupt as for other cardiovascular conditions, such as stroke or myocardial infarction, leg symptoms can severely affect patients' health status (their symptoms, functional status, and quality of life).2,3 Patients with PAD have a disproportionate burden of atherosclerotic risk factors and a significantly compromised health status.4 One-year cardiovascular event rates—including cardiovascular death, myocardial infarction, stroke, or other hospitalizations for atherothrombotic events—are estimated to be more than 21% in patients with PAD, as compared with 15% for coronary artery disease and stroke.5 Mortality rates are 15% to 30% within 5 years of diagnosis.6,7 Despite this, and the creation of performance measures to improve the quality of care,8 there are few assessments of compliance with these quality indicators in routine clinical care. Another barrier faced by patients with PAD is the lack of insurance coverage (at the time of conducting this study) for key PAD treatments, including supervised exercise therapy and smoking cessation programs. PAD not only affects patients' individual lives and those of their families; it also has a tremendous impact on society at large. The annual costs associated with vascular-related hospitalizations in PAD patients in the United States is estimated to exceed $21 billion.9 Regarding the documentation of patient-reported burden, PAD has received disproportionately less attention than coronary disease or stroke. As a comparison, a literature search for “heart disease” and “quality of life” and “stroke” and “quality of life” render 18 655 and 6885 results, respectively, whereas the terms “PAD” and “quality of life” result in only 781 hits (searched July 1, 2017).
Gap in Knowledge: Patients' Experiences With PAD Are Unknown
Because of its impact on patients' health status, one of the most important PAD treatment goals is to relieve symptoms and improve function. However, there is a paucity of real-world patient-centered outcomes data in PAD patients.6,10 When prior research efforts in PAD have examined health status, they have focused on generic quality of life outcomes11,12 and the safety or success of individual revascularization strategies (mortality, complications, and hemodynamic success rates) within the context of randomized clinical trials and procedural registries.13,14 The discordance between these prior studies and meaningful clinical outcomes is underscored by the poor correlation between indexes of technical success and patients' perceived health status.15 Moreover, prior procedure-based studies did not enroll patients treated with alternative strategies (such as medications or exercise)16 and did not systematically measure patients' disease-specific health status, leaving patients and providers uninformed about how different treatments are linked with expected health status outcomes and preventing them from making an informed, evidence-based decision about their PAD treatment.17 Studies that did focus on the association between PAD procedures and patients' health status were often small (<350 patients),18 were cross-sectional,19 included heterogeneous cohorts, or used generic instruments.20 Recent PAD trials have started to include disease-specific health status instruments as part of their outcome assessment21,22; therefore, more evidence should become available to patients in the foreseeable future. But until recently, only fragmented and limited approaches were available to measure patients' experiences with PAD, which may partly explain why formal quantification of patients' health status has not become a routine component of PAD treatment and care and why patients remain largely uninformed (because of the lack of systematic data) about what health status outcomes they should expect after undergoing different types of PAD treatment.
We designed the Patient-centered Outcomes Related to TReatment Practices in Peripheral Artery Disease: Investigating Trajectories (PORTRAIT) study to address these gaps in knowledge about quality of PAD care and health status outcomes of patients with PAD. Through PORTRAIT, we aimed to generate critical new knowledge to advance the quality of PAD treatment and directly addressed the following patient-centered questions: (1) “Given my personal characteristics, conditions, and preferences, what should I expect will happen to me?” (2) “What are my options and what are the potential benefits and harms of those options?” (3) “How can clinicians and the care delivery systems they work in help me make the best decisions about my health and health care?”
The study focused on patients referred to a vascular specialty clinic for new-onset or worsening claudication symptoms—a critical time for treatment decisions and, after which, the most dramatic changes in patients' health status are expected. We hypothesized that substantial variability exists in treatment patterns across providers and by patient characteristics (gender, racial, socioeconomic, psychological factors) and that this variability explains much of the variation in patients' health status outcomes. In phase I, we worked with patients and physicians familiar with PAD to design the PORTRAIT study. We tested the recruitment, enrolment, and follow-up process at 2 PAD specialty care clinics in the United States. In phase II, we further scaled the study to 10 US PAD specialty care clinics. The stakeholders agreed on the following aims for the PORTRAIT study: (1) We will examine variations in treatment by patient characteristics as a foundation for identifying potential disparities in care. (2) We will quantify patients' PAD-specific health status outcomes overall, and as a function of treatment and patient characteristics. (3) PORTRAIT will compare real-world PAD care against 4 endorsed PAD performance measures (statins, smoking cessation interventions, antiplatelets, and exercise therapy) to provide initial insights into the quality of PAD care and to support future, more widespread assessments and improvements in the quality of PAD care.8 (4) We will study the association between prescription of pharmacologic, revascularization, and supervised exercise therapies with PAD-specific health status outcomes. (5) PORTRAIT will develop prototype patient educational materials that summarize its findings in a patient-centered format.
We translated these aims into question format for ease of interpretation:
Do PAD treatments differ by patient characteristics, such as gender, race, or socioeconomic status? (2) Is early revascularization associated with more rapid improvements in patients' health status? (3) Do different doctors and health systems treat patients differently? (4) Are the variations in care observed in aims 2 and 3 associated with differences in patients' outcomes? and (5) Based on the results in aims 1 to 4, educational materials were created for future PAD treatments as a direct deliverable from the PORTRAIT phase II.
Participation of Patients and Other Stakeholders in the Design and Conduct of Research and Dissemination of Findings
Under phase I of the PORTRAIT registry, we collected meaningful input from relevant stakeholders experienced with PAD to prioritize outcomes, create screening logistics, and organize the optimal follow-up process. We created a physician and patient expert panel, each represented by 3 expert members, to advise and assist the research team in codesigning the study and prioritizing study outcomes. The individual members of these expert panels broadly represented the available PAD treatment modalities (exercise, medication, and invasive treatment).
Through selected patient focus groups with patients from different backgrounds, we also partnered with patients to define patient-centered data elements, research design, and relevant outcomes measures. In total, we organized 7 focus groups at the Saint Luke's Mid America Heart Institute, Kansas City, Missouri, and at Yale University Medical Center in New Haven, Connecticut. The topics we discussed with the patient focus groups were selected by the patient and physician expert panels (which are further explained below).
Under phase II of the study, all of the expert members who served on the panels described below advised and collaborated with the key research team to implement and execute the PORTRAIT registry.
The patient expert members comprised the following: Mr Bryant is a well-respected community leader and a past member of the city council (Kansas City 1983-1991). He has been diagnosed with PAD and has undergone several endovascular procedures for his symptoms. He was part of the expert panel during the phase I of the PORTRAIT study and continued to be part of the same patient expert panel that advised us throughout phase II of the study. Another member of our patient expert panel, Ms Legg, was already inspired to reach out to other members of the community to talk about this condition during phase I of the PORTRAIT study. She set up an information session on PAD in her local volunteer community circle; the session included a presentation and talk about PAD and its consequences by one of the specialists of Saint Luke's Mid America Heart Institute. She continues to be involved in PORTRAIT phase II. Our third member, who had been with us since phase I of the study, Mr Liedler, has been diagnosed with PAD for years and suffered a complication following a surgical revascularization procedure.
The physician expert members comprised the following: Dr Hirsch (†2017), a vascular medicine specialist and an internationally recognized authority in the field of PAD, was involved in pivotal PAD studies, including PARTNERS and REACH, and took up leadership roles on several national PAD guideline panels. Dr Tsai also served on our panel; he is an interventional cardiologist who is leading the efforts for the Veteran Affairs Cardiovascular Assessment, Reporting, and Tracking procedure-based registry that collects procedure-based information on outcomes following peripheral endovascular procedures across all VA hospitals. Last, Dr Aronow, who also served on our panel, is an interventional cardiologist who—in collaboration with colleagues at the University of Michigan—is leading the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. The consortium manages an ongoing procedure-based PAD registry, collecting procedural and health status outcomes following a peripheral vascular intervention.
The patient and physician expert panel met at least once per quarter and was consulted when urgent questions or issues arose. Both panels advised us throughout the study on aspects like study design and implementation. They also had a monitoring role and helped us interpret results such as enrollment feedback, and we presented (preliminary) results to them. Examples of questions were “How long do we allow for ankle-brachial index test results to be valid to count toward the screening criterion?” (question asked to the physician expert panel) or “What is the best way to explain the value of complete follow-up to participating patients?” (question asked to the patient expert panel).
Other stakeholders and experts that we engaged included clinicians and researchers in the field of vascular medicine, vascular surgery, and cardiology (past president of the American Heart Association, Mark Creager, MD; William Hiatt, MD; Mark Friedell, MD [†2016]) to serve on our observational safety monitoring board (OSMB), an expert in shared decision-making (Karen Sepucha, Massachusetts General Hospital), and our publications committee panel (3 site investigators voted by our research consortium as members).
The experts and stakeholders each brought unique expertise to the study. The physician expert panel advised the key research team on clinical aspects. The OSMB advised the team on human subjects–related matters throughout the study. The expert on shared decision-making advised us on assessing and interpreting study results related to decisional quality. The publication committee advised the research team on the quality and contents of the writing related to scientific publications and abstracts resulting from PORTRAIT. The patient expert panel represented the patient perspective throughout all phases of the study.
A complete overview of interactions with our stakeholders and the specific ways they were engaged in different phases of the study is provided in Table 1. The following are more specific examples of the ways the expert panels and stakeholders were involved throughout the different study phases.
Table 1
Overview of Stakeholder Engagement Interactions.
Example of Patient Stakeholder Engagement
In November 2014, we had our second in-person meeting with the patient expert panel. The goal of this meeting was to discuss the format and distribution plan for our patient educational tools. Different formats were going to be preferred (fact sheet, video pamphlets of patient stories, and a website). The website would be one way of distributing the materials; the different health systems of the participating sites would be distributing in other ways. We would also partner with organizations like the America Heart Association to help promote our website.
We also looked at the prioritization of outcomes that patients cared most about. We had them score outcomes of their PAD treatment on a range from 0 to 10. The top 4 outcomes they valued most were (1) importance of avoiding loss of toes or legs, (2) importance of decreased risk of heart attack and stroke, (3) importance of improving quality of life, and (4) importance of long-lasting treatment benefits.
Example of Physician Stakeholder Engagement
We consulted the physician expert panel mostly for its expertise on PAD and data collection efforts. Examples of topics we consulted the physician experts about included the validity of an ankle-brachial index (ABI) report over time and the difference between collecting raw data for the ABI measurement vs documenting the ratio only. In these examples, the panel established that the ABI report could not be older than 12 months, provided no PAD treatments would have been initiated, and the panel agreed that the sites would need to provide us with the raw data, rather than the calculated ratio.
Example of Observational Monitoring Board Engagement
In the fall of 2016, we worked closely with our OSMB, clinician expert members, and coinvestigators to streamline our publication process and to ensure that the abstracts and manuscripts being developed directly address our specific aims and that quality is ensured.
Methods
Study Design
PORTRAIT aims to describe the patient and treatment characteristics associated with patients' health status outcomes. We have thus designed a prospective, observational multicenter study with adequate power to examine unadjusted and multivariable-adjusted associations of patient and treatment characteristics with outcomes. Given our intent to describe the variability in routine PAD care, and the association of such variability with patient characteristics and outcomes, a prospective observational registry (as opposed to a retrospective one that would not have access to the important patient-centered characteristics [or outcomes] or a randomized trial where variations in care are more controlled) is the ideal study design for these questions. Below is an overview of the specific questions:
- Aim 1: Do PAD treatments differ by patient characteristics, such as gender, race, or socioeconomic status?
- Aim 2: Is early revascularization associated with more rapid improvements in patients' health status?
- Aim 3: Do different doctors and health systems treat patients differently?
- Aim 4: Are the variations in care observed in aims 2 and 3 associated with differences in patients' outcomes?
- Aim 5: Based on the results in aims 1 through 4, we created educational materials for future PAD treatments as a direct deliverable from the PORTRAIT phase II.
Although we studied only 10 select US outpatient clinics (which deployed different specialists, including vascular surgeons, interventional cardiologists, and vascular medicine specialists), we designed our research infrastructure to extend our study procedures to a wide variety of clinical settings in the future, including primary care practices and those of different specialists. In this study, it was not our intention to set up a screening study to capture the whole PAD population, including asymptomatic patients and patients seen at primary care settings.
Table 2
Inclusion and Exclusion Criteria for PORTRAIT.
Forming the Study Cohort
To be eligible, patients had to (1) be symptomatic with new-onset or recent exacerbations of exertional leg symptoms; (2) have a Doppler resting ABI ≤0.90 or a significant drop in postexercise ankle pressure of ≥20 mm Hg (ABI test results in preparation for the index visit or up to 1 year prior). Trained research assistants at 10 specialty clinics (vascular medicine, vascular surgery, cardiology), hereafter referred to as PAD clinics, identified potential participants from the appointment schedule, interviewed them at the clinic or by telephone, and obtained consent to participate before any invasive treatment or supervised exercise therapy had taken place.
Study Setting
Ten specialty PAD centers in the United States enrolled patients. A complete list and overview of sites is presented in Figure 1.
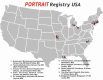
Figure 1
Overview of Sites.
Our study focused on examining the current patterns of care and quality of care that is provided to patients whose disease was severe enough to warrant treatment at a PAD specialty clinic, and who are often sicker than patients seen in primary care settings.23,24 We have chosen to collect data from a setting that sees these “sicker” patients, as these patients are—because of their symptoms and/or disability—the ones who will have the most room for improvement. Future intervention trials and quality of care programs will most likely be tested first in PAD specialty clinics, as this setting has the most burdened patients and these staff can implement quality of care improvements more readily through their experiences with earlier quality of care programs in coronary disease.
When patients seek care for symptoms of claudication, they may be seen by a variety of physicians in various contexts. Regardless of the setting, management of PAD should consist of cardiovascular risk management and symptom relief.25 Patients may be seen by their primary care physician, who works with them to pursue noninvasive options including claudication medications, cardiovascular risk management, and exercise therapy. Patients may also be referred for further evaluation and care to a PAD specialty care clinic. Specialties involved in the treatment of PAD may include surgery (vascular, cardiothoracic), vascular medicine, cardiology (general, interventional), and interventional radiology. Figure 2 summarizes this heterogeneous context and the potential clinical pathway that patients may follow, which ranges from noninvasive strategies (medications, exercise therapy, smoking cessation efforts) to invasive strategies (endovascular or surgical revascularization options).
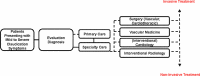
Figure 2
Clinical Pathway of Patients Presenting With PAD Symptoms.
Baseline Data Collection
Study coordinators at each site documented demographic, clinical, provider, and treatment information from patients' medical records and obtained information about patients' socioeconomic, psychosocial, and health status from standardized interviews using validated instruments.
We designed PORTRAIT to document the existence of potential disparities in health status outcomes regarding patients' age, sex, and race/ethnicity. We obtained information about age and sex from patients' medical records; patients self-reported their race/ethnicity. Race/ethnicity categories were captured using the revised 1997 Office of Management and Budget definitions.26 Additionally, from the medical records, we captured standard risk factors and comorbid conditions, as well as disease severity information, including patients' ABI results and noninvasive and invasive imaging results documenting burden and disease severity.
Information about adherence, including potential exclusions (medical, patient, system reasons), to 4 core American College of Cardiology (ACC)/American Heart Association (AHA) PAD performance measures8 were prospectively collected from patients' medical records. Data collectors were given a 1-month window following enrollment to abstract treatment information.
To create the educational tools, 7 focus groups were conducted with enrollees of the PORTRAIT studies and additional patients recruited from the community as well as those who fit PORTRAIT's enrollment criteria. A total of 60 patients participated in the focus groups; mean age was 70 years (range, 54-86 years); 26 patients were African American, 20 patients were Hispanic, and 14 were White; and 45 patients were female. A psychologist and a nurse led the groups; the 1-time sessions lasted for 90 minutes and included a structured discussion on formats, contents of the materials, and presentation style so that the framework to embed our PORTRAIT results for wider dissemination could be built.
Our study administration and data management were facilitated by a structured, integrated, internet-based, data collection infrastructure (Velos eResearch). Through this system, we tracked all screening information and recorded reasons for excluding patients who were screened but not enrolled; we also tracked basic socio-demographic information to monitor our enrollment targets for women and minorities. The data collection infrastructure complies with HIPAA regulations and uses the highest standards of data security and privacy.
Interventions
Our study focused on examining the current patterns and quality of usual care; thus, it did not implement an intervention or compare 2 interventions for the patients whom we enrolled.
Follow-up
From the feedback from patients we enrolled in phase I of PORTRAIT, we learned that PAD patients are very willing to share their health status information through regular phone interviews. We discussed with them what time points would be most relevant to them to best capture their experiences with PAD. Based on those discussions, we organized follow-up phone interviews at 3, 6, and 12 months, centrally administered by trained interviewers from the Mid America Heart Institute. The center contacted patients and administered the follow-up interviews using a standardized protocol. The measures collected in the follow-up interviews are listed in Figure 3. Our follow-up rates at 3 months were 89.2%, and at 6 and 12 months, they were 83.7% and 83.6%, respectively (see Figure 4).
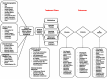
Figure 3
Overview of Potential Mediators/Moderators of Outcomes Following Presentation With New or an Exacerbation of PAD Symptoms.
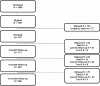
Figure 4
Flowchart With Overview of Screened and Enrolled Patients and Patients With Follow-up.
PORTRAIT was approved by the IRBs of all participating centers that recruited patients for this study. Written informed consent was obtained in 65% of the patients; in the remaining patients, telephone consent was obtained according to a protocol, which was approved by the participating sites' IRBs.
Study Outcomes
Primary Outcome
Health status assessments were performed at baseline and at 3, 6, and 12 months. The primary outcome for PORTRAIT is the health status information derived from the Peripheral Artery Questionnaire (PAQ), a 20-item, PAD-specific, multidimensional health status instrument.27 One item identifies the most symptomatic leg and the remaining 19 items are answered using variable Likert response scales with equidistant gradations of response categories. The PAQ quantifies the aspects of the conceptual model that we have used for our prior work in acute myocardial infarction and heart failure populations,28-30 and that we customized to capture the most salient aspects of patients' PAD-specific health status. Per this conceptual model, the following domains are key to measure disease-specific health status: physical function, symptoms, symptom stability, social limitation, treatment satisfaction, and quality of life as it relates to the functioning impacted by PAD. Applying a standardized scoring algorithm, a summary score (range, 0-100; higher scores indicate better health status) can be calculated that combines all scales except the treatment satisfaction and symptom stability scales.
Psychometric properties for the Walking Impairment Questionnaire,31 the Short Form 36,32 and exercise treadmill tests27 are excellent, with Cronbach α values between .80 and .94 and good convergent validity.
Secondary Outcome Measures
We supplemented the PAD-specific health status information from the PAQ with information from the San Diego Claudication Questionnaire (SDCQ),33,34 which discriminates between typical and atypical exertional leg symptoms and has a 91% sensitivity and 68% specificity for clinician-diagnosed claudication.35 We assessed generic health status with the EQ-5D questionnaire,36 a widely used generic instrument that describes health states along 5 dimensions (mobility, self-care, usual activities, pain or discomfort, and anxiety/depression) and a visual analogue scale that rates individuals' perception of their overall health. The EQ-5D Index, a single summary index, can be used to generate utilities for potential economic analyses. We chose this instrument because of its widespread use, its brevity, and the potential to use its data in health-economic analyses. Finally, several psychosocial assessments were completed using well-validated measures, including depression (Patient Health Questionnaire-8),37 anxiety (Generalized Anxiety Disorder 7-item scale),38 stress (Perceived Stress Scale),39 and social support (ENRICHD Social Support Inventory),40 as well as a thorough documentation of socioeconomic factors (income, health insurance, difficulties accessing care, financial concerns).41,42 A complete overview of the constructs measured throughout the PORTRAIT study is provided in Figure 3.
PORTRAIT phase II also produced the foundation for education tools derived from a diverse group of patients with treatment effects documented on a clear 1-year timeline. These materials were made by patients and PAD experts so that future patients with PAD can easily understand what other patients who underwent similar treatments experienced regarding their health status in the year following PAD treatment.
Analytical and Statistical Approaches
Overall analytic considerations underlying our statistical analyses are as follows: To address confounding and selection biases inherent in observational data, and to analyze the hierarchical data (patients treated by different individual providers within different sites), we used 3-level hierarchical regression models to permit adjustment for potential confounding and to account for clustering within provider and site, to estimate patient-, provider-, and site-level effects on outcomes. In addition, hierarchical models allow for robust estimation of individual provider and site outcomes by “shrinking” estimates from providers/sites with small sample sizes toward the mean.
For dichotomous outcomes (eg, adherence to performance measures; aim 3), the variance parameters can be transformed into more interpretable median odds ratios, which denote the median relative difference in outcome rates between 2 patients with the same covariates seen by 2 randomly selected providers or sites.43 This expresses the variability among providers/sites in a metric that can be directly compared with the odds ratios/relative risks of other model covariates.
Specifically, for our aims, we conducted the following analyses: For aim 1 (Do PAD treatments differ by patient characteristics, such as gender, race, or socioeconomic status?), we conducted a series of ANOVAs with PAQ domain scores as the dependent variables and baseline demographics as independent variables. To compare referral to invasive treatment vs noninvasive treatment by demographics, we conducted chi-square tests. Subsequently, we conducted a hierarchical, multivariable logistic regression model with a random factor for site and the referral to invasive treatment as a dependent variable. We included the following independent variables in the model: age, sex, race, health insurance, marital status, new vs exacerbation of symptoms, typical symptoms, lesion site, claudication severity, claudication duration, smoking, surgical treatment, prior endovascular treatment, diabetes, history of coronary artery disease, chronic kidney disease, antiplatelet use, referral to exercise therapy, ABI, PAQ baseline summary score, problem solving and decision-making as the preferred role for shared decision-making, any direct anatomical visualization, and claudication medication use. For aim 2 (Is early revascularization associated with more rapid improvements in patients' health status?), we calculated PAQ change scores for each domain at each follow-up time point (3, 6, and 12 months) and conducted ANOVAs with the PAQ change scores as dependent variables, and sex and race as independent variables. Then, we conducted generalized linear model repeated measurements modeling with PAQ summary scores over time included and invasive treatment as an independent variable. We tested the interaction time × invasive treatment. We proceeded with constructing a hierarchical linear regression model to predict 1-year PAQ summary scores. We used a random forest algorithm to weigh 50 baseline variables such that a selection (those with >5% increase in mean square error) could be retained. We calculated the explained variance of that model and also tested interactions with the variables in the model and treatment (invasive vs noninvasive). For aim 3 (Do different doctors and health systems treat patients differently?), we examined adherence rates to 4 performance measures (statins, antiplatelet therapy, supervised exercise therapy, and smoking cessation support) among those eligible by demographics (age, sex, race) using chi-square tests and ANOVAs, as appropriate. We also combined 2 of the measures (statin and antiplatelet therapy) as well as all 4 of the measures and defined them as perfect medical care and perfect care, respectively, and did the same comparisons as with the individual performance measures. We then constructed a hierarchical logistic regression model with a random effect for site, to evaluate whether sex, race, and age were associated with perfect medical care and perfect care, respectively. Finally, we calculated median odds ratios for these 2 latter models to evaluate site variability. For aim 4 (Are the variations in care observed in aims 2 and 3 associated with differences in patients' outcomes?) we evaluated whether patients' PAQ health status scores differed by receipt of perfect medical care or perfect care vs those who did not receive such care (independent variables), using ANOVAs for all PAQ domains (summary, quality of life, social limitations, physical limitations, symptoms, and symptom stability scores as well as their change scores as dependent variables, across all time points).
We conducted all analyses using SAS v9.4 (SAS Institute) and IVEware (Institute for Social Research). We evaluated statistical significance at a 2-sided significance level of <.05.
We based power calculations for PORTRAIT on PORTRAIT's main objective (aim 2): To quantify patients' PAD-specific health status outcomes overall, and as a function of treatment and patient characteristics. Based on this aim, we hypothesized that revascularization will be associated with more rapid, and larger, improvements in health status as compared with noninvasive options, and that these benefits will vary by age, gender, baseline health status, smoking cessation, minority race, and depressive symptoms.
Upon designing PORTRAIT, we estimated to enroll 840 patients across 9 sites in the United States over 18 months. We estimated these numbers assuming that most of the centers would screen 160 eligible baseline patients and that 75% (120) would participate in the study. Some centers had lower enrollment targets owing to their lower historic patient volume. Assuming a conservative loss to follow-up of 20% (based on our initial goal of 840 patients), we calculated power using a projected sample size of 672. As most of the baseline variables will be analyzed dichotomously, these power calculations are applicable to most of the variables specified in our hypothesis. We based our calculations on the smallest detectable effect size (α = .05; β = .80) using an SD in 1-year summary PAQ scores of 27 (based on our ALEVE study) for both unadjusted and adjusted multivariable models.16 The proportion of variance in the variable of interest explained by other variables in the model is expressed as the square of the partial correlation [r]. Because a meaningful difference in Summary PAQ scores is 8 points,16,27 adequate power is available for a wide range of potential prevalence rates for the independent (predictor) variables. For example, in a scenario in which we would observe a revascularization rate of 50%, when comparing health status outcomes of patients who were treated invasively vs those who were managed noninvasively, we would have the power to detect a change between 5.8 and 6.3 on the change summary PAQ scores at each time point, even in multivariable models that explain 20% to 40% of the variation in PAQ scores.
Conduct of the Study
Because study procedures were implemented and tested in 2 centers under phase I of the study, phase II mainly consisted of scaling the study to 10 study sites and the study procedures that were developed and refined under phase I. Patients were asked to participate upon first visiting the PAD clinic, before treatment was started or altered. Study coordinators and investigators at each center obtained informed consent (either in person or over the phone) and performed the baseline interview at the outpatient clinic.
While epidemiologic findings in community-dwelling individuals reveal that PAD is equally prevalent among men and women,44-46 women are typically underrepresented in clinical populations because their PAD is not as easily recognized as in men, often owing to atypical symptoms.47 Epidemiologic findings from the community may not translate to the specialty setting regarding gender breakdown, as it has been reported that PAD symptoms in women are underrecognized.9 Based on prior screening efforts under phase I, we expected to enroll between 35% and 40% women; through the use of screening logs, we ensured that the proportion of enrolled women was reflective of the proportion presenting to such clinics.
Estimates of PAD prevalence among minorities vary (eg, ∼23% for African Americans; ∼14% for Hispanics),48 but we sought to include a minimum of 30% minorities, as mandated by Congressional legislation.
Data Quality Monitoring
Our statistician programmed data queries to check data quality; these queries were run on a weekly basis and the reports were sent to site coordinators. We required that all outstanding data queries be resolved before sites could receive reimbursement for the time their site study coordinator used to screen and enroll each patient. In addition to the queries, we monitored follow-up rates and missing data on a weekly basis. Other measures we put in place to ensure quality data were the following: If some patients were not able to finish the complete interview, we offered to complete it on the following day. In case this was not possible, we had carefully arranged the order of the questionnaires to capture the most important information first (eg, PAD-specific health status). To further maximize follow-up rates, our follow-up center made multiple attempts to reach each missing patient, including calling the patient's contacts or the primary physician, based on information we collected during the baseline interview. If none of these strategies proved successful, we then mailed the interview questions to the patient's home or work address, also recorded at baseline, or worked with the enrolling site to identify a future scheduled visit that coincided with the follow-up window. A comparison of those who enrolled vs those who did not enroll in the study, as well as a comparison of those who did or did not have 1-year follow-up, is provided in Supplemental Files No. 2 and No. 3.
Results
Screening Results
A total of 1995 patients were screened (Figure 4). The participating sites routinely measured the ABI. Of those who presented with abnormal ABIs, 1007 (50.5%) met the PORTRAIT study criteria. Of the patients who were eligible, 797 (79.1%) consented to participate. No major differences existed between those who were enrolled and those who were eligible but did not enroll (n = 211; due to refusal [n = 133] or unreachable by telephone [n = 77]), except that a higher representation of African Americans occurred among those enrolled.
Baseline Patient Characteristics
Characteristics of enrolled patients are presented in Table 3. Overall, the mean age was 68.6 ± 9.7 years and 41.9% were female. A total of 27.6% were non-White. About 1 in 5 had experienced a myocardial infarction in the past; 56.7% were former smokers and another 30.3% were current smokers. About a third of enrolled patients had a history of a peripheral vascular intervention, 40.5% had diabetes, and the mean body mass index was 29.5.
Table 3
Baseline Characteristics of PORTRAIT Patients for the Overall Population.
The following PAD characteristics were noted: About half of the patients presented with moderate symptoms of claudication according to the Rutherford classification. Their mean ABI was 0.67 ± 0.19. Of all enrolled patients, 40.2% presented with new-onset symptoms of PAD, of which most were typical claudication symptoms (87.9%). For 65.7% of participants, their interventional cardiologist was their main PAD care provider, and for 18.6% of participants, their general cardiologist was their main PAD care provider; the remainder of the participants were seen by vascular surgeons, vascular medicine specialists, interventional radiologists, or allied professionals. The enrolling specialist also served as the main provider for the PAD management that was documented under PORTRAIT.
Missing Data
The average number of missing data fields per patient for the patient interview data was 0.54. Values for at least 1 study variable (out of the 241 collected variables per patient) were missing in 21.7% of patients. The clinical data fields for the mandatory data elements were complete in all patients. Additional peripheral diagnostic information and laboratory values were abstracted up to a year before and 1 month following enrollment if they were available. We intended to capture real-world practices; therefore, diagnostic tests or laboratory draws were not mandatory data elements.
Follow-up Data
An overview of patients who died, were too ill, refused to participate, or were lost to follow-up over the course of 1 year is presented in Figure 4.
Specific Aims
Aim 1: Do PAD Treatments Differ by Patient Characteristics, Such as Gender, Race, or Socioeconomic Status?
Will patients who have a worse health status upon diagnosis be more likely to receive invasive treatment for their PAD (surgical or percutaneous revascularization) as compared with patients who have a better health status upon diagnosis?
At the 3-month follow-up, we documented that 141 patients (141/693; 20%) received an invasive treatment strategy (endovascular or surgical revascularization). In line with our hypothesis, we observed that patients who received invasive treatment had lower health status scores compared with patients who were referred to a noninvasive strategy. As for our primary outcome measure, the PAQ—a disease-specific health status instrument—patients who received an invasive treatment, compared with those who received a noninvasive treatment, had consistently lower scores on the baseline summary score (42.9 ± 19.9 vs 49.0 ± 22.4; P = .003), the baseline quality of life score (43.2 ± 24.2 vs 51.0 ± 26.2; P = .001), and the physical limitations score (28.6 ± 22.3 vs 37.6 ± 25.6; P < .0001).
A complete overview of comparisons of patient characteristics by referral to invasive treatment is provided in Table 4. Note that the primary treatment referral strategy (invasive vs noninvasive treatment) is defined as referral to treatment within the first 3 months following the PAD workup. Treatments offered beyond 3 months were deemed not to be reflective of the primary treatment strategy (based on input from the physician expert panel) and were not the focus of this analysis. A multivariable model that predicts referral to invasive treatment is presented in Figure 5. Lesion site, ABI, and disease severity were associated with referral for invasive treatment.49 Patients with proximal disease (vs distal disease) had 2.5 increased odds of receiving invasive treatment; patients with severe (vs mild) claudication had a >2-fold odds of receiving invasive treatment, and a higher ABI value was associated with a 12% decreased odds per 0.1 unit increase. Despite the univariate association between lower baseline health status PAQ summary scores and invasive treatment, health status did not reach statistical significance in the multivariable model as a predictor for referral to invasive treatment.
Table 4
Results for Aim 1: Patient Characteristics Associated With Referral to Invasive Treatment.
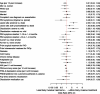
Figure 5
Multivariable Predictors of Referral to Invasive Treatment.
Will elderly patients be less likely to receive invasive treatment for their PAD as compared with younger patients?
The mean age of patients receiving invasive treatment was 67.5 ± 9.4 years vs 69.2 ± 9.6 years in those who did not receive invasive treatment (P = .052).
Will women be less likely to receive invasive treatment for their PAD as compared with men?
The rate of invasive treatment was not different between men (55.3%) and women (44.7%); P = .32.
Will minority-race patients be less likely to receive invasive treatment for their PAD?
The rate of minority-race patients receiving invasive treatment was 40/186 or 21.5%; the rate of White patients receiving invasive treatment was 101/507 or 20%, P > .05.
Aim 2: Is Early Revascularization Associated With More Rapid Improvements in Patients' Health Status?
Will patients with PAD who are treated invasively have more rapid and larger improvements in health status changes over time as compared with those who are treated noninvasively?
Do their symptoms and health status differ at 1 year?
The magnitude of change in patients' PAD-specific health status scores was greater in patients who underwent invasive treatment vs those who were not referred to invasive treatment, at 3 (PAQ summary change score, 25.6 ± 20.8 vs 16.4 ± 23.0; P < .001), 6 (PAQ summary change score, 29.6 ± 23.8 vs 17.4 ± 23.3; P < .001), and 12 (PAQ summary change score, 28.2 ± 23.7 vs 17.0 ± 24.4; P < .001) months follow-up. Similar patterns were observed for patients' quality of life change scores, physical limitation change scores, and symptom and symptom stability change scores (results of univariate comparisons reported in Table 5).
Table 5
Results for Aim 2: Health Status Outcomes Over Time by Invasive vs Noninvasive Treatment Referral.
In addition, repeated measurement analyses integrated all PAQ summary scores over time. The mean scores were compared by invasive treatment. The interaction effect that tested time × treatment was not significant (Figure 6).
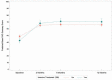
Figure 6
Mean PAQ Summary Scores Over Time by Treatment Type (Invasive Treatment vs Noninvasive Treatment).
Will the degree of improvement in patients' health status be smaller for certain subpopulations of patients?
Comparisons by sex and by minority race are presented in the following paragraphs.
Table 6
Comparisons of Patient Characteristics and Outcomes by Sex.
Table 7 reports the patient characteristics and health status outcomes over time by race (univariate comparisons). PAQ summary scores are consistently lower in minority-race patients at each follow-up time point. These observations are also noted for quality of life, social limitations, physical limitations, symptoms, and symptom stability. As for the magnitude of health status changes over time, minority-race patients had smaller improvements in their health status as compared with patients of White race, although the comparisons were not always statistically different. For a complete overview of scores, see Table 7.
Table 7
Comparisons of Patient Characteristics and Outcomes by Race.
For the multivariable modeling to predict 1-year health status (PAQ summary scores), we considered 50 variables and weighted them using a random forest algorithm (Figure 7). We retained 10 of those variables (all >5% increase in mean square error) in a linear regression model that predicted 1-year PAQ summary scores (Figure 8a). To examine the incremental value of 12 additional clinical variables (eg, ABI), we built a second model (Figure 8b). Figure 8a explained 29% of the variance in 1-year health status. Shorter symptom duration, higher baseline PAQ summary scores, and male sex were associated with better outcomes at 1 year (Figure 8a). Depressive symptoms, bilateral disease, chronic lung disease, and sleep apnea were linked with worse outcomes. Adding 12 clinically relevant variables minimally improved the explained variance (31%; Figure 8b). No significant interactions with the variables and PAD treatment modality were present in either model.

Figure 7
Importance Plot of Variables for 1-Year PAQ Summary Outcomes.
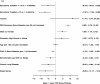
Figure 8a
Core Prediction Model for 1-Year PAQ Summary Score Outcomes.
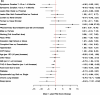
Figure 8b
Core Prediction Model + Additional Clinical Important Variables Predicting 1-Year PAQ Summary Outcomes.
Aim 3: Do Different Doctors and Health Systems Treat Patients Differently?
What are physicians' adherence rates with 4 recently endorsed PAD performance measures (use of statins, aspirin, smoking cessation, referral to exercise therapy)?
More specifically, will patients with PAD be less likely to be referred for exercise, as opposed to pharmacologic or revascularization therapies when seen by some doctors and health systems, as opposed to others?
We studied adherence to the 4 official PAD performance measures for this aim.8 A total of 90.3% participants received an antiplatelet (n = 709) following their PAD evaluation, 83.5% received a statin (n = 666), and only 1.8% (n = 14) were referred to a formal PAD exercise program. A total of 80% of the smokers received smoking cessation advice or smoking cessation support (n = 184). Based on the number of eligible performance measures received, most patients (61.9%) received 2 performance measures (statin and antiplatelet therapy); only 4.6% (n = 37) received perfect care (defined as adherence to all eligible performance measures).50 As adherence to all 4 performance measures was so low, given the low rates of exercise therapy referrals, we also looked at the quality of care by defining perfect medical care as adherence to the 2 medication performance measures (statin and antiplatelet), in addition to looking at perfect care (adherence to all 4 measures).
When looking at demographic predictors of receiving perfect care for these 4 measures, we observed that age was inversely related (although not statistically different) with receiving perfect medical care (odds ratio [OR], 0.82; CI, 0.66-1.01; P = .059). Other covariates—sex and race—were not predictive of receiving perfect care.
When calculating the median odds ratio (MOR) as an indicator of site variability, we derived a MOR of 5.43 for this model (P < .0001), indicating significant site variability in the adherence to all 4 performance measures (ie, the rates of perfect care) across participating sites. A MOR estimates the average relative difference in 2 hypothetically identical patients who receive perfect care when treated at 2 random sites.51
When looking at demographic predictors of receiving perfect medical care (antiplatelet therapy and a statin), we observed that men were more likely to receive perfect medical care compared with women (OR, 1.51; CI, 1.07-2.14; P = .0185). Other covariates—age and race—were not predictive of receiving perfect medical care.
When calculating the MOR as an indicator of site variability, we derived a MOR of 2.13 for this model (P = .10), indicating that there was no significant site variability in the adherence to the 2 performance measures across participating sites.
Aim 4: Are the Variations in Care Observed in Aims 2 and 3 Associated With Differences in Patients' Outcomes?
Will patients whose providers failed to prescribe evidence-based treatments (eg, exercise, statins, antiplatelet drugs) have a poorer 1-year health status as compared with those who receive these treatments?
We compared health status scores over time based on receiving perfect medical care vs not receiving perfect medical care (receiving platelet therapy and a statin; Table 8) and based on receiving perfect care vs not receiving perfect care (receiving all eligible performance measures; Table 9).
Table 8
Health Status Outcomes Over Time by Receiving Perfect Medical Care (Prescribing Antiplatelet/Statin Therapy).
Table 9
Health Status Outcomes Over Time by Receiving Perfect Care (Prescribing All Eligible PAD Performance Measures).
Health status scores measured at each time point were higher for those who received perfect medical care (antiplatelet therapy and statin therapy) vs those who did not receive perfect medical care (Table 8) at each time point, except for the 12-month outcomes. This was the case for the PAQ summary scores, the quality of life scores, and the PAQ symptoms scores. For patient health status, no significant differences occurred in the magnitude of change over time between the groups that received perfect medical care vs those that did not (Table 8).
Health status scores were the same for participants who received perfect care (all eligible performance measures) and those who did not receive perfect care. However, higher scores were noted for the 3-month PAQ summary score and 3- and 6-month PAQ symptoms score in those who received perfect care vs those who did not receive perfect care. The magnitude of change in health status scores was different for 3-month symptom changes (perfect care group had greater health status benefits). No other differences were noted. A complete overview of results is presented in Table 9.
Aim 5: Based on the Results in Aims 1 to 4, We Will Create Educational Materials for Future PAD Treatments
As a direct deliverable from the proposed PORTRAIT phase II study, we created patient education tools with a team of experts consisting of patients with PAD and PAD specialists.
In consultation with the patient experts and using input from patient focus groups, we developed a series of educational materials that will be further developed into future decision aids. In the focus groups, we obtained rich input on barriers and facilitators to adequate decision-making. In addition, we presented the patients with prototypes of educational tool formats and got their input on how we could improve the materials and whether we would need to amend them to reflect patients' needs and preferences. The formats that we discussed were a fact sheet, patient vignettes, and decision cards. The facilitators of good decision-making were process variables (eg, doctor–patient trust, being provided with options, transparency), transfer of knowledge, and sensitivity to preferences. Among the barriers to good decision-making that were mentioned were lack of knowledge or unclear presentation of information, system-related barriers (time constraints, health care model, fee-for-service model), and cost of care.
The feedback from the patients on the materials we presented included explicit instructions on how we can tailor the materials to meet patients' needs. Recurrent recommendations were a diversity of material types and that materials need to be available in advance of the clinical visit, while in the waiting room, and in the doctor's office. The materials need to serve the purpose of knowledge transfer, conversation starter, preparation for visit, as a reminder, as an agent for reflection and change, and as inspiration to know what questions to ask. Examples of the discussion cards and patient vignettes are provided in Figures 9 and 10. These materials will eventually be grouped onto a website.

Figure 9
Example of Patient Vignettes Developed With Input From Patient Experts.

Figure 10
Example of Discussion Cards.
To disseminate the patient-education tools derived from the PORTRAIT study, we are working with the AHA to further update our materials. We will then use AHA's dissemination channels to make further available our patient-centered tools for prospective patients with PAD. We have had 2 conversations with the director of the vascular disease section at the AHA to partner with AHA to disseminate the PORTRAIT findings through patient-centered materials through the AHA network. Finally, we are preparing to develop an individualized prediction kit for our educational materials. This will occur through a research proposal that is being prepared for submission through https://grants.nih.gov/grants/guide/pa-files/PA-16-282.html [link no longer works].
Table 10
ClinicalTrials.gov Tables: (a) Patient Flowchart; (b) Baseline Analysis Population Description; (c) Outcomes Data; (d) Adverse Events Reporting.
Discussion
Context for Study Results
Unlike other cardiovascular disorders, PAD has received limited scientific attention. PORTRAIT extends the field of PAD research in several important directions. First, we have described treatment patterns over the year after diagnosis and compared them with PAD performance measures of quality.6,8 In the past, when variations in the quality of PAD care have been explored, they have been performed cross-sectionally,52 in primary care settings with heterogeneous groups of patients,53 or they have been procedure based.54 It was therefore unknown how risk factor management and treatment decisions were made for those being evaluated for new-onset or exacerbated PAD symptoms—critical states for reconsidering optimal therapy—or how treatments evolve over time. By quantifying the variability in treatment for PAD patients, and how they change over the first year of treatment, we provided novel insights into the quality of PAD care.
Randomized trial evidence has shown that endovascular treatment and exercise are equally beneficial over time for patients with aorto-iliac disease.55,56 In the PORTRAIT registry, we consecutively enrolled 797 patients with new symptoms or an exacerbation of PAD symptoms from 10 clinics in the United States. In the PORTRAIT study, about 1 in 5 patients were referred to undergo an invasive treatment strategy (endovascular or surgical revascularization). Patients who were referred for invasive treatment had experienced more severe claudication and had lesions that were more amenable to receive invasive treatment. In a multivariable prediction model, the independent predictors of referral for invasive treatment were lesion site and claudication severity.
While adherence to the individual PAD performance measures8 was relatively high for statins (90%) and for antiplatelet therapy (84%), the adherence to the PAD exercise program referral was very low. Barely 2% of the patients were referred to a formal exercise program for patients with PAD, underscoring the need to further investigate the barriers to prescribing this evidence-based performance measure. When looking at whether patients received the 2 medications that lower their PAD and cardiovascular risk (statin and antiplatelet therapy), only 62% received such dual risk management strategy, and only 4.6% received perfect care (all 4 performance measures). Older age was inversely associated with receiving perfect care; women were less likely to receive perfect care. The rates of adherence to the performance measures varied considerably across the 10 study sites.50
A second contribution of PORTRAIT is the prospective collection of information on patients' health status (their symptoms, function, and quality of life). Despite current guidelines that stress the importance of symptom relief and improved quality of life as primary goals for PAD treatment,25 there are no large, representative studies systematically evaluating the magnitude of health status changes associated with different PAD management strategies.18 By examining treatment variability and its association with patient-centered outcomes using the validated, disease-specific Peripheral Artery Questionnaire,27 we provided a systematic, prospective assessment of PAD patients' disease-specific health status and its association with treatment.
Those who were referred for invasive treatment experienced larger health status benefits over time, as compared with those who were not referred for invasive treatment. These patterns were largely mirrored in people's quality of life, physical limitations, and symptom change scores, which all showed larger benefits for those who were referred to invasive treatment.
When looking at health status differences by patient characteristics, women had consistently lower PAD-specific health status scores than men, which was also true for minority-race vs White patients. Men and women experienced the same magnitude of health status gains over time.
Furthermore, we noted a risk-treatment paradox (healthier patients received better care) when looking at the receipt of PAD evidence-based care: Patients with higher health status scores were more likely to receive PAD evidence-based care (perfect medical care: statin and antiplatelet therapy) as opposed to those with lower health status scores. Health status scores were also higher throughout the follow-up assessments in those who received evidence-based care. On the other hand, the receipt of evidence-based PAD care did not predict the magnitude of health status gains over time; however, the study was underpowered for this comparison, owing to the small numbers of patients who received all evidence-based care metrics.
The third benefit to health care systems relates to the direct translation of our findings to patients with PAD. By having developed educational materials based on the PORTAIT findings, patients with PAD can—for the first time—be informed about their expected health status outcomes.
A fourth advance from PORTRAIT has been our ability to lay the foundation for future comparative effectiveness studies of the most common treatment strategies, including medications, smoking cessation, exercise, and revascularization. By incorporating a range of outcomes, including patients' health status, and by complementing our research efforts in 10 US centers, we observed a wide variation in treatment (eg, the availability of and access to supervised exercise programs for PAD). These data will form the basis for future comparative effectiveness research work.
A fifth important contribution of PORTRAIT has been to identify subsets of the population (eg, age, gender and racial groups, potentially mediated by psychological or socioeconomic factors) at risk for poorer processes of care and outcomes.
A final advance of the PORTRAIT study has been that patients have been and continue to be engaged as active advocates and experts of their disease throughout the process of the study design, implementation, and, ultimately, the analysis and translation of its findings.
Generalizability of the Results
We did not intend to set up a screening study to capture the whole PAD population, including asymptomatic patients and patients seen at primary and specialty care settings. Instead, our study focuses on examining the current patterns of care and quality of care that is provided to patients whose disease is severe enough to warrant treatment at a PAD specialty clinic and who are often sicker than patients seen in primary care settings.24 Clinically, this represents a critical moment for treatment decisions in the process of managing PAD patients. Moreover, we have chosen to collect data from diverse settings that are seeing these “sicker” patients, as these patients are the ones who will have the most room for improvement. Therefore, the more rapid health status improvements observed for invasive treatment in our registry should be interpreted against that context, as probably patients with a heavier disease burden were prioritized to receive invasive treatment. With this type of design, we acknowledge that the quality of life outcomes documented in the PORTRAIT study are not representative of the entire PAD population, as many individuals with reduced blood flow in their lower extremities may not have been diagnosed or are not being seen in a specialty care setting. Wider efforts are, however, also needed to reach patients with PAD who are not seen in the specialty care setting, and to find ways to engage underserved populations that may experience barriers to accessing the care they need for their PAD.
Implementation of the Study Results
This is not applicable because the study was observational rather than interventional.
Subpopulations Considerations
This is not applicable because the study was not designed to compare alternative treatments for PAD.
Study Limitations
Patient preferences about whether an invasive vs conservative management of PAD was chosen were not accounted for in the referral patterns for invasive treatment and may help explain some of the variability in referrals for invasive treatment.
We selected the enrollment sites; a more nationwide, generalizable representation of PAD specialist centers, including centers geographically situated in the West or the Central United States, may be desirable in the future, as more condensed versions of patient-centered outcomes registries will be developed.
We acknowledge that multivariable models will need to be further optimized to reduce the risk of selection bias and residual confounding in our models and to cross-validate our work with other existing cohorts. Propensity matching, stratification, or adjustment are techniques that we will consider to additionally address confounding in future iterations of our work.57 The magnitude of bias owing to unobserved follow-up will be further evaluated through sensitivity analyses (eg, assigning worst possible outcomes to patients with missing data) and inversely weighting observations based on the propensity to participate in follow-up. Finally, as we only recruited from a select set of centers that represent only a fraction of patients with PAD, we will seek ways to partner with stakeholder organizations and use our initial experiences to design and execute wider, national data collection initiatives that capture the patient-centered experience in a systematic and ongoing way.
Future Research
Our next steps following the PORTRAIT study are 2-fold: (1) We need to retain the core elements from PORTRAIT that will be able to capture patients' experience in larger, ongoing quality registry initiatives such as the ACC's NCDR-PVI registry or the Society for Vascular Surgery's registry (this will allow us to document how variability in care is associated with the patients' experience and to document value care from the patients' perspective on a larger scale across the nation); and (2) the patient outcomes by treatment documented in PORTRAIT and summarized in our patient education materials need to be tested in a prospective setting. The PORTRAIT education materials will be ideal to be tested in a randomized controlled trial design to evaluate how they are able to better engage and inform patients as they make PAD treatment decisions. A more systematic information and engagement process in the medical decision-making for PAD treatment will allow patients to weigh all treatment options, including the currently underused exercise therapy, and evaluate how these options align with their treatment preferences. As we further materialize the prediction models to be derived from the PORTRAIT study, our educational materials can function as a foundation for future, evidence-based decision-making aids. Individualized prediction models can help estimate the expected health status benefits given a patient's clinical and demographic variables and given their choice of treatment (invasive vs noninvasive options).
Conclusions
Collectively, the PORTRAIT study is a multicenter observational follow-up study that has unique serial health status data available on patients who were presenting to PAD specialty clinics with new or worsening PAD symptoms. It has rich information about patient characteristics, PAD treatments, and patient-centered outcomes. As we identified core data elements to collect in wider national registries that patients find important, as well as gaps in the implementation of evidence-based PAD care, PORTRAIT will help redefine current national PAD registries so that it captures the patients' experience.
One of the core findings that requires immediate attention is that supervised exercise therapy for PAD, despite being a Class I recommendation, is hardly available to patients. Fewer than 2% of our enrolled population had access to this primary treatment strategy. Availability of formal programs and reimbursement of these strategies are key areas to demand attention for, to allow patients to get access to, this form of treatment.
In addition, disseminating our work to patients to educate them about their options for PAD management and how these options translate to health status outcomes will empower patients to ask their provider relevant questions and will promote discussions that will enable them to make more treatment decisions that align with their preferences.
The information collected in PORTRAIT will help accomplish our long-term goal of creating a disease-management model that will facilitate the adoption of evidence-based treatments and allow patients to have productive interactions with a team of PAD specialists about what treatments would be preferable for them. With this model, we hope to be able to move away from the current state of fragmented PAD care and usher in an era of more interactive, evidence-based, patient-centered care.
References
- 1.
- Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics- 2016 update: a report from the American Heart Association. Circulation. 2016;133:e38-e360. [PubMed: 26673558]
- 2.
- Smolderen KG, Pelle AJ, Kupper N, Mols F, Denollet J. Impact of peripheral arterial disease on health status: a comparison with chronic heart failure. J Vasc Surg. 2009;50:1391-1398. [PubMed: 19958988]
- 3.
- Hallin A, Bergqvist D, Fugl-Meyer K, Holmberg L. Areas of concern, quality of life and life satisfaction in patients with peripheral vascular disease. Eur J Vasc Endovasc Surg. 2002;24:255-263. [PubMed: 12217289]
- 4.
- McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women's health and aging study. Circulation. 2000;101:1007-1012. [PubMed: 10704168]
- 5.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197-1206. [PubMed: 17374814]
- 6.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463-e654. [PubMed: 16549646]
- 7.
- Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026-3049. [PubMed: 8941154]
- 8.
- Olin JW, Allie DE, Belkin M, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). Circulation. 2010;122:2583-2618. [PubMed: 21126978]
- 9.
- Mahoney EM, Wang K, Cohen DJ, et al. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes. 2008;1:38-45. [PubMed: 20031786]
- 10.
- Poku E, Duncan R, Keetharuth A, et al. Patient-reported outcome measures in patients with peripheral arterial disease: a systematic review of psychometric properties. Health Qual Life Outcomes. 2016;14:161. [PMC free article: PMC5121983] [PubMed: 27881127]
- 11.
- Whyman MR, Fowkes FG, Kerracher EM, et al. Is intermittent claudication improved by percutaneous transluminal angioplasty? A randomized controlled trial. J Vasc Surg. 1997;26:551-557. [PubMed: 9357454]
- 12.
- Wu A, Coresh J, Selvin E, et al. Lower extremity peripheral artery disease and quality of life among older individuals in the community. J Am Heart Assoc. 2017;6(1):e004519. doi:10.1161/JAHA.116.004519 [PMC free article: PMC5523635] [PubMed: 28108464] [CrossRef]
- 13.
- Murphy TP, Ariaratnam NS, Carney WI Jr, et al. Aortoiliac insufficiency: long-term experience with stent placement for treatment. Radiology. 2004;231:243-249. [PubMed: 15068949]
- 14.
- Duda SH, Pusich B, Richter G, et al. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation. 2002;106:1505-1509. [PubMed: 12234956]
- 15.
- Long J, Modrall JG, Parker BJ, Swann A, Welborn MB III, Anthony T. Correlation between ankle-brachial index, symptoms, and health-related quality of life in patients with peripheral vascular disease. J Vasc Surg. 2004;39:723-727. [PubMed: 15071432]
- 16.
- Safley DM, House JA, Laster SB, Daniel WC, Spertus JA, Marso SP. Quantifying improvement in symptoms, functioning, and quality of life after peripheral endovascular revascularization. Circulation. 2007;115:569-75. [PubMed: 17242281]
- 17.
- Kalbaugh CA, Taylor SM, Blackhurst DW, Dellinger MB, Trent EA, Youkey JR. One-year prospective quality-of-life outcomes in patients treated with angioplasty for symptomatic peripheral arterial disease. J Vasc Surg. 2006;44:296-302; discussion 302-303. [PubMed: 16814976]
- 18.
- Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg. 2011;53:1410-1421. [PMC free article: PMC3541824] [PubMed: 21334172]
- 19.
- Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) program. Vasc Med. 2008;13:15-24. [PubMed: 18372434]
- 20.
- Dumville JC, Lee AJ, Smith FB, Fowkes FG. The health-related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Pract. 2004;54:826-831. [PMC free article: PMC1324915] [PubMed: 15527608]
- 21.
- Nordanstig J, Taft C, Hensater M, Perlander A, Osterberg K, Jivegard L. Improved quality of life after 1 year with an invasive versus a noninvasive treatment strategy in claudicants: one-year results of the Invasive Revascularization or Not in Intermittent Claudication (IRONIC) Trial. Circulation. 2014;130:939-947. [PubMed: 25095886]
- 22.
- Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376:32-40. [PubMed: 27959717]
- 23.
- McDermott MM, Kerwin DR, Liu K, et al. Prevalence and significance of unrecognized lower extremity peripheral arterial disease in general medicine practice. J Gen Intern Med. 2001;16:384-390. [PMC free article: PMC1495229] [PubMed: 11422635]
- 24.
- Brevetti G, Oliva G, Giugliano G, Schiano V, De Maio JI, Chiariello M. Mortality in peripheral arterial disease: a comparison of patients managed by vascular specialists and general practitioners. J Gen Intern Med. 2007;22:639-644. [PMC free article: PMC1852923] [PubMed: 17354043]
- 25.
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;135(12):e726-e779. [PMC free article: PMC5477786] [PubMed: 27840333]
- 26.
- Recommendations from the Interagency Committee for the Review of Racial and Ethnic Standards to the Office of Management and Budget Concerning Changes to the Standards for the Classification of Federal Data on Race and Ethnicity. Office of Management & Budget; 1997. https://clintonwhitehouse2.archives.gov/OMB/fedreg/directive_15.html
- 27.
- Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301-308. [PubMed: 14760329]
- 28.
- Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333-341. [PubMed: 7829785]
- 29.
- Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103-2110. [PubMed: 19001034]
- 30.
- Green CP, Porter CB, Bresnahan DR and Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245-1255. [PubMed: 10758967]
- 31.
- Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142-152.
- 32.
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health economics. 1993;2:217-227. [PubMed: 8275167]
- 33.
- McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159:387-392. [PubMed: 10030313]
- 34.
- McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease. Associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606. [PubMed: 11585483]
- 35.
- Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101-1109. [PubMed: 1474406]
- 36.
- EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199-208. [PubMed: 10109801]
- 37.
- Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163-173. [PubMed: 18752852]
- 38.
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. [PubMed: 16717171]
- 39.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-396. [PubMed: 6668417]
- 40.
- Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23:398-403. [PubMed: 14646785]
- 41.
- Rahimi AR, Spertus JA, Reid KJ, Bernheim SM, Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297:1063-1072. [PubMed: 17356027]
- 42.
- Smolderen KG, Spertus JA, Nallamothu BK, et al. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. 2010;303:1392-1400. [PMC free article: PMC3020978] [PubMed: 20388895]
- 43.
- Larsen K, Petersen JH, Budtz-Jorgensen E, Endahl L. Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56:909-914. [PubMed: 10985236]
- 44.
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738-743. [PubMed: 15262830]
- 45.
- Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185-1191. [PubMed: 17543683]
- 46.
- Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329-1340. [PubMed: 23915883]
- 47.
- McDermott MM, Greenland P, Liu K, et al. Sex differences in peripheral arterial disease: leg symptoms and physical functioning. J Am Geriatr Soc. 2003;51:222-228. [PubMed: 12558719]
- 48.
- Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163:1469-1474. [PubMed: 12824097]
- 49.
- Pokharel Y, Wang J, Hiatt W, et al. Abstract 18984: patterns and predictors of invasive treatment referral in peripheral artery disease: insights from the PORTRAIT study. Circulation. 2016;134:A18984.
- 50.
- Saxon JT, Smolderen K, Spertus J, Gosch K, et al. Abstract 17323: adherence to ACC/AHA treatment guidelines for PAD across treatment sites: insight from the PORTRAIT registry. Circulation. 2016;134:A17323.
- 51.
- Merlo J, Chaix B, Ohlsson H, Beckman A, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290-297. [PMC free article: PMC2566165] [PubMed: 16537344]
- 52.
- Kroger K, Schwertfeger M, Pittrow D, Diehm C. Management of patients with peripheral arterial disease in primary care: a cross-sectional study in Germany. Int J Clin Pract. 2010;64:875-884. [PubMed: 20584220]
- 53.
- Blacher J, Cacoub P, Luizy F, et al. Peripheral arterial disease versus other localizations of vascular disease: the ATTEST study. J Vasc Surg. 2006;44:314-318. [PubMed: 16890860]
- 54.
- Cambou JP, Aboyans V, Constans J, Lacroix P, Dentans C, Bura A. Characteristics and outcome of patients hospitalised for lower extremity peripheral artery disease in France: the COPART Registry. Eur J Vasc Endovasc Surg. 2010;39:577-585. [PubMed: 20303804]
- 55.
- Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139. [PMC free article: PMC3374869] [PubMed: 22090168]
- 56.
- Reynolds MR, Apruzzese P, Galper BZ, et al. Cost-effectiveness of supervised exercise, stenting, and optimal medical care for claudication: results from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) trial. J Am Heart Assoc. 2014;3:e001233. doi:10.1161/JAHA.114.001233 [PMC free article: PMC4338709] [PubMed: 25389284] [CrossRef]
- 57.
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-2281. [PubMed: 9802183]
Acknowledgment
Research reported in this report was [partially] funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#CE-1304-6677) Further information available at: https://www.pcori.org/research-results/2013/treatment-and-patient-characteristics-affecting-health-status-patients
Appendix
#1 Key Concepts Used Throughout the Report (PDF, 75K)
Suggested citation:
Smolderen KG, Spertus J, Safley D, et al. (2019). Treatment and Patient Characteristics Affecting the Health Status of Patients with Peripheral Arterial Disease -- The PORTRAIT Study. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/4.2019.CE.13046677
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Review PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): Overview of Design and Rationale of an International Prospective Peripheral Arterial Disease Study.[Circ Cardiovasc Qual Outcomes....]Review PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): Overview of Design and Rationale of an International Prospective Peripheral Arterial Disease Study.Smolderen KG, Gosch K, Patel M, Jones WS, Hirsch AT, Beltrame J, Fitridge R, Shishehbor MH, Denollet J, Vriens P, et al. Circ Cardiovasc Qual Outcomes. 2018 Feb; 11(2):e003860.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Adherence to Guideline-Recommended Therapy-Including Supervised Exercise Therapy Referral-Across Peripheral Artery Disease Specialty Clinics: Insights From the International PORTRAIT Registry.[J Am Heart Assoc. 2020]Adherence to Guideline-Recommended Therapy-Including Supervised Exercise Therapy Referral-Across Peripheral Artery Disease Specialty Clinics: Insights From the International PORTRAIT Registry.Saxon JT, Safley DM, Mena-Hurtado C, Heyligers J, Fitridge R, Shishehbor M, Spertus JA, Gosch K, Patel MR, Smolderen KG. J Am Heart Assoc. 2020 Feb 4; 9(3):e012541. Epub 2020 Jan 24.
- Predictors of Revascularization in Lower-Extremity Peripheral Artery Disease: Insights From the PORTRAIT Study.[J Endovasc Ther. 2023]Predictors of Revascularization in Lower-Extremity Peripheral Artery Disease: Insights From the PORTRAIT Study.Pokharel Y, Kokkinidis DG, Wang J, Gosch KL, Safley DM, Spertus JA, Mena-Hurtado C, Smolderen KG. J Endovasc Ther. 2023 Jun 13; :15266028231179574. Epub 2023 Jun 13.
- Review Treatment Strategies for Patients With Peripheral Artery Disease[ 2013]Review Treatment Strategies for Patients With Peripheral Artery DiseaseJones WS, Schmit KM, Vemulapalli S, Subherwal S, Patel MR, Hasselblad V, Heidenfelder BL, Chobot MM, Posey R, Wing L, et al. 2013 May
- Treatment and Patient Characteristics Affecting the Health Status of Patients wi...Treatment and Patient Characteristics Affecting the Health Status of Patients with Peripheral Arterial Disease -- The PORTRAIT Study
Your browsing activity is empty.
Activity recording is turned off.
See more...
