This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Among people with neurogenic bladder (NB), urinary tract infections (UTIs) are the most common cause of morbidity, emergency department visits, and rehospitalizations. As such, UTIs and the urinary symptoms that characterize them represent a significant burden among people with NB.
Objectives:
For specific aim 1 (SA1), we aimed to develop and validate a patient-centered, patient-reported outcome (PC-PRO) urinary symptom questionnaire for individuals with NB with intermittent catheterization (USQNB-IC) due to spinal cord injury, spina bifida, or multiple sclerosis. For SA2, we aimed to develop and pilot test a Self-management Protocol using Probiotics (SMP-Pro) to reduce the severity, frequency, and impact of urinary symptoms. For SA3, we aimed to estimate the strength of the associations between successful implementation of the SMP-Pro and urinary symptoms, bladder inflammation, and the urine microbiome.
Methods
- (SA1) Using a patient-centered qualitative approach with over-the-phone focus groups and semistructured interviews, we developed the USQNB-IC.
- (SA2) Development of the SMP-Pro also used a blended patient-centered and evidence-based iterative approach. We then conducted an 18-month prospective single-arm quasi-experimental pre-post trial of the SMP-Pro (6 months each of urinary symptom observation: baseline, intervention, and washout) of self-management of urinary symptoms with intravesical Lactobacillus rhamnosus GG (LGG, Culturelle) using the USQNB-IC for weekly monitoring and the SMP-Pro during the intervention phase.
- (SA3) Safety (adverse events), tolerability, and effectiveness (trends of frequency, severity, and impact of symptoms) were analyzed over time in participants in the pre-post trial of SMP-Pro. Symptom burden and adverse events were primary outcomes. Secondarily, urine was collected in 26 participants to assess bladder inflammation and bacterial load.
Results:
For SA1, we enrolled 103 (96 adults, 7 children) participants. After completion of the alpha version of the USQNB-IC, 29 items resulted and were validated (face, content, divergent, and convergent validation) with a national sample of 581 people. In the pilot trial of SMP-Pro, “cloudy/foul-smelling urine” was identified as the best self-management trigger for the following reasons: (1) These symptoms are not considered indicative of a UTI according to evidence-based guidelines;1,2 and (2) in our SA1 national sample, “cloudy” and “foul-smelling” urine were the 2 most commonly reported urinary symptoms (SA2). Using the validated USQNB-IC to monitor urinary symptoms and the SMP-Pro to guide intravesical LGG instillation, we found that 1 to 2 doses of intravesical LGG instilled within 30 hours in response to cloudy/foul-smelling urine (1) was safe and well-tolerated in our adults with NB who use IC; (2) reduced urinary symptom burden; and (3) did not alter bladder inflammation or bacterial load (SA3). Further, there is preliminary evidence of safety and tolerability in children.
Conclusions:
The USQNB-IC is a PC-PRO measure for monitoring of urinary symptoms among people with NB who use IC. In this first assessment of self-instilled intravesical LGG using the SMP-Pro approach in people with NB who use IC, intravesical LGG was safe and well-tolerated. Among adults, study results suggest that intravesical LGG may be effective at reducing urinary symptoms and does not alter bladder inflammation or bacterial load of the urine. There is preliminary evidence that intravesical LGG is safe to use in children with NB who use IC.3 Data are not sufficient to determine effectiveness in children.
Limitations:
This was a first-in-human intervention trial of intravesical LGG for urinary symptoms, and as such, the scope and breadth of the study intervention and study population were strictly controlled. As this was not a randomized controlled study, definitive conclusions regarding effectiveness of the intervention are not possible to make. The results support continued research to expand the participant population and to further explore optimal dosing.
Background
Magnitude of Urinary Tract Infections and Problems With Their Diagnosis
Neurogenic bladder (NB) is associated with a disproportionately high risk of genitourinary (GU) complications, including bladder and kidney infections,4-7 calculi,8,9 and bladder cancer,5,10 among others. (For a list of the abbreviations used in this report, please refer to Appendix A.) While NB can result from any trauma or disease of the brain or spinal cord, people with spinal cord injury (SCI) and spina bifida (SB) are nearly universally affected. Urinary tract infections (UTIs) were historically the most common cause of death for people with SCI/SB7, and while early mortality due to UTI and subsequent kidney failure has declined with improved prevention and management, UTIs remain the most common hospital-acquired infection worldwide. For individuals with SCI/SB and NB, it is the most common infection, secondary condition, cause for emergency department visits, and infectious cause of hospitalization.5,11-17
This worldwide public health problem has staggering economic impact and potential for human suffering. In a recent published systematic review on the attributable cost of catheter-associated UTIs in the hospital setting, the estimated cost per episode was in excess of $1000 per infection ($876 to $10 197 based on patient population and hospital setting).18 Further, it is estimated that there are 561 677 catheter-associated UTIs occurring in the hospital setting annually, and these UTIs are responsible for 8205 deaths.14 These impacts do not include the personal suffering or time lost from gainful employment.
Diagnostic Challenges of UTIs
Despite its prevalence, attempts to ameliorate UTIs among people with NB due to SCI or SB are stymied by long-standing diagnostic challenges that arise from evidence gaps regarding “gold standard” diagnostic test criteria (urinalysis and urine culture) that have lower sensitivity and specificity for UTIs in this population. A high prevalence of chronic inflammation leading to persistence of white blood cells (WBCs) in the urine of patients with NB12,13,19,20 confounds the utility of using WBC count, pyuria, and leukocyte esterase (LE) as inflammatory biomarkers specific to UTI. Nitrites in urine indicate the presence of only specific (but not all) bacteria, many of which are present to a greater extent in the urine of people with SCI or SB; and people with NB have a high prevalence of asymptomatic bacteriuria.21,22 These physiologic changes render the gold-standard diagnostic tests less useful for identifying UTIs in individuals with NB due to SCI or SB.
Authoritative Guidelines for UTI Diagnosis Among People With NB
These diagnostic challenges persist despite coordinated efforts by the National Institute on Disability and Rehabilitation Research,23 Agency for Healthcare Research and Quality,24 the European Association of Urology (EAU)1 with other European and Asian urologic societies,1,25 and the Infectious Diseases Society of America (IDSA).2 Each has generated guidelines for the prevention of catheter-associated UTIs among people with NB without promoting the diagnosis of UTI.1,2 All guidelines note their lack of high-quality evidence support, and each calls for research addressing the challenge of UTI diagnosis.26
Among contemporary guidelines for diagnosis of catheter-associated UTI in adults and children due to NB are the US-based IDSA Guidelines for diagnosing catheter-associated UTI (regardless of type of catheter or presence/absence of NB)2; the European-based EAU Guidelines on Urological Infections1; and a systematic review by Madden-Fuentes et al27 used for children with NB due to SB (who primarily use intermittent catheterization [IC]).27 The 2017 EAU Guidelines largely rely on the 2009 IDSA Guidelines, so diagnostic criteria are identical for both. Further, both the IDSA and EAU guidelines require at least 1 symptom indicative of UTI, including new onset or worsening fever, rigors, altered mental status, malaise, lethargy, flank pain, costovertebral angle tenderness, acute hematuria, pelvic discomfort, dysuria, urgent or frequent urination, suprapubic pain or tenderness, increased spasticity, autonomic dysreflexia, and sense of unease.1,2 In contrast, Madden-Fuentes et al27 require at least 2 symptoms (from the list of fever >38 °C, abdominal pain, new back pain, new or worse incontinence, pain with catheterization or urination, and malodorous/cloudy urine)—of these, only “fever” matches the IDSA/EAU symptom list. Further, the IDSA and EAU specifically state that “cloudy or malodorous urine” is not considered a symptom of catheter-associated UTI among people with NB,1,2 whereas Madden-Fuentes does allow consideration of malodorous/cloudy urine. However, at least 1 additional symptom is required to meet the symptom criteria of UTI.27 This is relevant as cloudy and foul-smelling urine is the most frequently reported urinary symptom among people with NB. This factor likely contributes to patient and clinician uncertainty of decision-making regarding urinary symptoms, especially cloudy and foul-smelling urine.
Gaps in Measurement of Urinary Symptoms Indicative of UTI
Urinary symptom questionnaires exist for other patient populations with lower urinary tract dysfunction (eg, patients with prostate disease, urge incontinence, overactive bladder, and/or interstitial cystitis).28-38,38,39 Examples of these instruments include the International Prostate Symptom Score;30 the International Continence Society (ICS)31 male questionnaire and the short-form male questionnaire for patients with benign prostatic hyperplasia32; the Incontinence Impact Questionnaire33 for urinary incontinence; the Chronic Prostatitis Symptom Score34; the O'Leary-Sant Symptom Index35; the Urgency Perception Score;36 the Indevus Urgency Severity Scale;37 the Overactive Bladder Symptom Score38; the core Lower Urinary Tract Symptom score;38 and the Urogenital Distress Inventory-6 to determine types of symptoms associated with urinary tract dysfunction.39
Two questionnaires that are urinary specific have been validated in those with SCI with NB: The Dutch Short-Form (SF)-Qualiveen measure focuses on the urinary-specific quality of life in 4 domains: bother with limitations, fears, feelings, and frequency of limitations. In the development of this instrument, consumers with SCI and urologic symptomatology assessed content validy.40 The second questionnaire is the Neuropathic Bladder Symptom Score (NBSS), which was created to broadly measure urinary symptoms and their consequences in patients with acquired or congenital NB; however, unlike our work, this questionnaire is clinician centered and not patient centered. In addition, the NBSS is not focused on urinary symptoms related to UTI.41 In sum, the Dutch SF-Qualiveen and NBSS use a patient-reported outcome approach but not the patient-centered, patient-reported outcome (PC-PRO) approach that we use in our work, and although these measures are focused on urinary issues, they do not have the same UTI-related focus as our instrument. In contrast, to assure the primacy of the patient experience during the development of our instrument, which focuses on UTI-related urinary symptoms, we asked patients questions relating to their lived experience with urinary symptoms, not just those determined by clinicians or researchers, with the goal of fully representing the patient experience.42
Self-management Using Probiotics
Because of the frequency with which urinary symptoms and UTIs are experienced among this population (supported by our previous work43 and that of others demonstrating the potential benefit of intravesical Lactobacillus),44 we designed the first-in-human intravesical Lactobacillus probiotic clinical trial. However, there are evidence gaps contributing to confusion around the potential use of probiotics for UTIs. For example, a Cochrane review titled “Probiotics for preventing urinary tract infection in people with neuropathic bladder” was conducted and published in 2017.45 Because there were no studies on the use of other probiotics, such as Lactobacillus rhamnosus GG (LGG, Culturelle), the authors of the Cochrane review examined use of nonpathogenic Escherichia coli (the most common type of bacteria that causes UTIs) as a potential “probiotic.” In these 3 studies, similar to ours, intravesical inoculation of bacteria into the bladder was assessed.45 Two of these studies used intravesically instilled E coli 83972 and saline in a population with SCI,46,47 and in 1 study, intravesical E coli 83972 was assessed in people with voiding dysfunction due to neurological causes.48 The overall quality of these studies was poor, with high attrition due to patient participant burden, frequent complications, and reporting bias.45 Furthermore, although the E coli used in these studies was considered “low virulent,” E coli generally remains the most common uropathogen, thereby increasing risk to consumers and cost due to the highly controlled environment that is required to produce “low-virulence” E coli. Therefore, readers need to remain observant of the broad use of the term “probiotics” in the scientific literature, which can lead to potential confusion among consumers, clinicians, researchers, and other stakeholders. The intervention in our study uses a widely available Lactobacillus probiotic and is based on preliminary evidence, as described previously.
Based on our pilot data43 and the evidence found, we determined that Lactobacillus would be a potentially beneficial agent for urinary health. Compounds secreted by various Lactobacillus strains (against uropathogenic E coli [UPEC]) have been shown in animal models to inhibit pathogen growth, induce UPEC membrane stress, and/or downregulate proteins critical for pathogen attachment.49 Further, multiple Lactobacillus strains have demonstrated antiadherence properties against selected uropathogenic bacteria (similar to gastrointestinal effects50-52). Of the Lactobacillus strains, Lactobacillus rhamnosus has arguably been the most frequently studied. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 supernatants have been shown to strongly inhibit UPEC growth and downregulate promoter activity of the major subunits of type 1 and P fimbriae, which are important adherence factors for the bacteria in the urogenital tract.46 Lactobacillus rhamnosus GR-1 has been shown to reverse the UPEC-induced downregulation of nuclear factor-κB, and the same strain can disrupt E coli biofilms.53-55 Further, indigenous Lactobacillus has been shown to significantly reduce kidney and bladder Proteus mirabilis counts in animal models.53 And in vitro, 6 Lactobacillus strains were each found to have similar antimicrobial activities against 4 different uropathogens.56 Further, Lactobacillus inhibits selected bladder cancer cell proliferation, is cytotoxic to other bladder cancer cells, and induces apoptosis—all in the presence of mammalian cells.57 Thus, preclinical results support our selection of LGG for this intervention.
Study Objectives
In this project, we aimed to fill these multiple gaps by (1) creating and validating a patient-centered, patient-reported urinary symptom questionnaire for individuals with NB with IC (USQNB-IC58); and (2) developing and assessing a new intravesical LGG intervention to manage the most commonly reported urinary symptoms before a UTI occurs. Specifically, the USQNB-IC fills the urinary symptom (related to UTI) measurement gap, while the LGG intervention fills a gap related to use of more traditional probiotics (as opposed to nonpathogenic or less-pathogenic bacteria) for urinary symptom management and potential UTI prevention.
Specific Aims
The specific aims (SAs) of this study were as follows:
- SA1: Develop and validate a urinary symptom questionnaire for individuals with NB due to SCI, SB (children and adults), and multiple sclerosis (MS). This was the first patient-centered questionnaire for this population (see Appendix B).
- SA2: Develop and pilot test a Self-management Protocol using Probiotics (SMP-Pro) to reduce the severity, frequency, and impact of urinary symptoms.
- SA3: Estimate the strength of the association between successful implementation of the SMP-Pro and urinary symptoms, bladder inflammation, and the urine microbiome.
For SA2 and SA3, we compared urinary symptom outcomes during 6 months of usual care followed by 6 months of intravesical LGG, as guided by the SMP-Pro.
Participation of Patients and Other Stakeholders
Patients and stakeholders were an integral part of the study team, from writing the proposal to participating in dissemination activities. During proposal preparation, we conducted several focus groups involving people with SCI and SB to better understand patients' interests in self-management of urinary symptoms and the potential use of probiotics when experiencing urinary symptoms to avoid progression to UTI (these were separate and distinct from the focus groups in SA1). We received feedback on the aims proposed for the study, and the issues raised were incorporated into the design. For instance, the focus groups helped define inclusion/exclusion criteria for compatible (eg, oral vitamin C supplements, use of cranberry, etc) and incompatible (eg, prophylactic irrigation of the bladder with gentamycin) practices. Indivudials with SCI and SB suggested that educational materials on LGG and the instillation process be created, as well as technical materials for study participants to share with their health care providers. These suggestions were incorporated into the proposal and later developed once we received the award.
Once awarded, we identified 6 individuals with mixed backgrounds and experiences (ie, consumer experts) who were added to the research team: 3 individuals with SCI, 2 caregivers of children with SB, and 1 individual with SB. All consumer experts were involved in SA1 of the study, which included participation in conference calls to develop the focus group guide and to facilitate participation in focus groups with patient participant interviewees. One of the consumer experts led analysis of the focus groups transcripts using NVivo 10 (QSR International).59 This involved being trained and learning the skills necessary to use the qualitative research software and then taking the lead to conduct this portion of the project.
Consumer experts worked closely with the research team in developing the SMP-Pro and the educational materials (patient-friendly instructions on how to instill probiotic into the bladder) used during the intervention phase. Focus groups of consumers and clinicians separately, and then together, were conducted iteratively to develop a beta version of the SMP-Pro. Then, 6 consumers used the SMP-Pro on a trial basis to specifically assess its usability as well as the use of intravesical LGG under the envisioned circumstances. The most important discoveries of activation testing were that individuals who use closed catheter systems do not experience the symptom of malodorous urine and that malodorous and cloudy urine typically occur together. As a result of this feedback and insight, the SMP-Pro was changed so that either symptom, malodorous or cloudy urine, would equally trigger the self-treatment intervention. Further modifications based on this testing included changing the time frame between instillations from 24 to 30 hours (to accommodate realistically instilling at various times of the day), and a more rapid reassessment after the second instillation. The 4-hour time frame after the second instillation was set to quickly identify people who had worsening of symptoms, so that in this situation, a potential infection would not be allowed to persist only due to study protocol issues.
Consumer experts taught participants how to use the SMP-Pro. Three of the consumer experts were trained to oversee participant instruction and training on how to instill the LGG probiotic into their bladders before beginning the intervention phase of the study. In addition, the consumer experts followed up with their assigned patient participants monthly during the intervention phase to confirm that participants' questions were quickly and thoroughly answered, and to help maintain participants' engagement in the study. Upon study completion, several consumer experts have continued to be involved with development of dissemination materials, including creation of a more patient-friendly design for the USQNB-IC and videos informing patients about the questionnaire.
In addition to supporting participants in the research project, one of the consumer experts initiated a prospective effort aimed at contextualizing study findings based on individuals' experiences with reimbursement for urinary catheters. Consumer experts conducted semistructured, hour-long interviews by phone with 4 patients and 2 caregivers. In addition to patient interviews, they also interviewed the CEO of a catheter manufacturing company, a catheter provider (distributor) representative, and a disability advocacy activist. The overarching theme across interviews was the lack of a clear pathway in the US health care system to assure that patients have access to the catheter type, quality, and quantity that optimizes their physical and psychosocial health. The health care practitioners who provided catheter prescriptions were perceived as fulfilling that function only, with no help in selecting catheter type and providing no additional guidance or system navigation. Patient participants described experiences of uncertainty upon enrolling with an insurance plan, as to whether the catheter type that best suited their needs would actually be reimbursed. Several patients described recourse to their catheter providers (distributors) to provide guidance on filling this information gap, and reliance on the provider to educate them regarding possible, acceptable alternatives if their preferred catheter was no longer covered by insurance. The provider/distributor representative who was interviewed also described the mediating role of the distributor team, working to meet patients' needs within their available (reimbursed) options.
Our consumer and advocacy partner organization, United Spinal Association (USA), was an integral partner throughout the study. USA facilitated outreach to 581 individuals with SCI, SB, and their caregivers nationally, thereby assisting in the validation process of the beta version of the USQNB-IC. This was accomplished by posting information on the USA website and in their monthly newsletter, and by sending targeted emails to those who had self-identified in their membership profile as having SCI or SB. The same approach was taken for participant recruitment for SA2 and for our dissemination activities.
Methods and Results by Specific Aim
Study Overview
In the following sections we describe the methods and results by study aim. First, we describe the development of the urinary symptom questionnaire, which includes qualitative and iterative components of a patient-centered approach to patient-reported outcome development. This is followed by a description of the USQNB-IC's validity testing, using responses from a large national sample. Second, we describe the development of the self-management intervention protocol (ie, the SMP-Pro) using an iterative participatory design approach. With the USQNB-IC validated and the SMP-Pro complete, we then describe the methods and results for our pilot trial of the protocol. Although urinary symptom burden (measured using the USQNB-IC) was the primary effectiveness outcome, we also report on safety (using adverse events [AEs] and serious adverse events [SAEs] as outcomes), tolerability, and changes in the microbial ecosystem.
Methods: SA1 Development and Validation of the USQNB-IC
We aimed to develop and validate a urinary symptom questionnaire for individuals with NB due to SCI, SB (children and adults), and MS, focusing on urinary symptoms. This was the first patient-centered questionnaire for this population (see Appendix B).
For SA1 questionnaire development, participants for focus groups were recruited locally and nationally with the assistance of USA and included people with SCI, SB, or MS who managed their NB with IC. We conducted 11 focus groups/interviews with 24 participants (14 SCI patients, 5 SB patients, and 5 caregivers). Consumer experts and research staff developed a semistructured interview guide iteratively and collaboratively. The range of participants per group/interview was 1 to 3 (three 1-participant interviews, four 2-participant groups, and four 3-participant groups). All focus groups were recorded, transcribed, and analyzed with NVivo10.46
Using grounded theory methods, 2 of our consumer experts worked collaboratively to reconcile the codes, categories, and themes identified in participants' shared experiences and perspectives. Preliminary findings were iteratively fed back to the larger consumer expert group to verify interpretation of what the focus group participants had shared. The alpha version of the USQNB-IC was a product of this process. We iteratively continued to modify the alpha USQNB-IC to accommodate this input until a version of the USQNB-IC emerged that included all urinary symptoms that were relevant to patients, caregivers, and health care providers with respect to NB, urinary symptoms, and UTI.
This resulted in the beta USQNB-IC, which included only items that were endorsed by at least 2 focus groups; items that the focus group patient participants identified as redundant were reviewed by the clinical panel of 4 clinicians with expertise in this domain to distinguish items more clearly or combine/reword items to keep the USQNB-IC specific and relevant, without sacrificing face and content validity. The beta USQNB-IC, with 29 items, was then deployed for national validation with a diverse national sample of 600 people with NB due to SCI and SB (300 adults and 300 children or their caregivers). Our consumer partner USA helped in recruiting for this national sample.
Participants in the National Sample Validation of the USQNB-IC
With the assistance of our consumer partner USA, we recruited nationwide participants with confirmed diagnoses of NB who manage their bladder with IC and who had a history of UTIs to complete the USQNB-IC online at 1 time point using SurveyMonkey. Each item was presented as a query about whether the respondent had experienced it during the past year (yes/no), with 3 additional required responses: average annual frequency (0-365 days); average severity (usually not at all severe; usually somewhat severe; usually severe; always very severe); and average impact on, or importance in, daily life (rarely affects my actions or decisions to go about my daily life; sometimes affects my actions or decisions to go about my daily life; usually affects my actions or decisions to go about my daily life; always affects my actions or decisions to go about my daily life).
Validity Testing of the USQNB-IC for SA1
To explore convergent and divergent validity, we also completed nationwide data collection using the USQNB-IC among 3 control groups: individuals with NB who did not have a history of UTIs (n = 49); individuals with chronic mobility impairments (neither SCI nor SB) and without NB (n = 46); and those with no mobility impairment, no NB, and no history of UTIs (n = 64). Once data collection was completed, we referred to the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) report60 to complete the validation process of the USQNB-IC (Table 1). Given the early stage of this instrument, we examined both construct and criterion validity (which COSMIN defined as including divergent and convergent validity) with distributions of scores (total and item endorsements) for the target population of individuals with NB who manage their bladders with IC, and also with data from divergent controls.
Table 1
COSMIN Criteria and How Each Criterion Was Assessed in This Study.
Changes to the Study Protocol for SA1
There were no changes to the original protocol for developing this measure.
Results: SA1 Development and Validation of the USQNB-IC
The following tables show descriptive results for each of our 3 responding diagnostic groups, followed by the descriptive data from our 3 “control” groups: individuals with NB who do not have a history of UTIs (n = 49), individuals with chronic mobility impairments (neither SCI nor SB) and without NB (n = 46), and those with no mobility impairment, no NB, and no history of UTIs (n = 64). Then, we describe the validity and reliability results for the USQNB-IC based on these respondents.
The results are presented in 2 parts in our published paper, “Preliminary validation of a urinary symptom questionnaire for individuals with neuropathic bladder using intermittent catherization: a patient-centered patient reported outcome” in PLoS One; an additional bayesian analysis is reported in this paper (see Appendix C).58
Table 2 demonstrates descriptive data of the 581 participants who completed the national survey. Table 3 summarizes descriptive data of the participants who completed the survey for purposes of convergent and divergent validity assessment.
Table 2
Descriptive Data of National Sample Participants With SCI or SB or Their Caregivers.
Table 3
Descriptive Data of Participants in Comparison Groups.
USQNB-IC Validity Evidence and Reliability for SA1
The COSMIN report identifies 4 key psychometric or measurement domains on which a health related PRO should be characterized:
- Reliability (minimization of measurement error, internal consistency or interrelatedness of the items, and maximization of variability that is due to “true” difference between levels of the symptoms across patients)
- Validity (content, reflection of the construct to be measured; face, recognizability of the contents as representing the construct to be measured; structural, the extent to which the instrument captures recognizable dimensions of the construct to be measured; and criterion, association with a gold standard)
- Cross-cultural validity
- Interpretability of scoring
Table 4 presents the summary validity evidence based on COSMIN.
Table 4
COSMIN Reliability Evidence: Patient Groups.
Additional content and face validity evidence arises from an examination of endorsement rates across the 3 groups of nationally representative patients with NB in our sample. Table 5, excerpted from Tractenberg et al,58 provides the endorsement rates (number of respondents identifying having experienced the symptom within the past year/total number of respondents in that group) by persons with NB for each symptom on the USQNB-IC. Endorsement rates were similar for individuals with SCI and SB, whereas endorsement by item was slightly different for the caregivers.
Table 5
Validity Evidence: USQNB-IC Item Endorsement by Group (Nationwide Validation).
The similarities of endorsement rates across groups supports content and face validity as well as some evidence for convergent validity. It also suggests that NB—which was the defining feature of the focus groups from which the symptoms were derived—does have a set of “signs and symptoms” that people with NB may experience.
Divergent and Convergent Validity of the USQNB-IC
Table 6 presents the endorsement rates across our “control” groups. These groups were recruited to evaluate the prevalence of USQNB-IC urinary symptoms among other groups (not those with SCI or SB) with mobility impairments without NB, those with NB but who do not have frequent UTIs, and those without mobility impairment, NB, or frequent UTIs.
Table 6
Convergent/Divergent Validity Evidence: Item Endorsement in Percentage, by Group, With the Number of Respondents for Whom Endorsement Was Relevant/Possible in Parentheses.
The last row in the table shows that the average number of endorsed items was 5.8 of 29 for the controls overall; this is less than half of the average for the study population groups (Table 5). The highest average (SD) number of endorsed items was in the group without mobility impairments and without NB (7.0 [4.4] items). The lowest average (SD) number of endorsed items (4.6 [4.3] items) was for those with mobility impairments who did not have NB.
There was agreement in the 3 most frequently endorsed items (muscle aches, increased urgency, and fatigue) among the control groups shown in Table 6. None of the endorsement rates for these items was as high as it was for any of the NB patient groups; the most striking difference between these control group endorsement rates and those from our national sample for the survey is for those with NB who have not experienced any (many) UTIs. Their rate of endorsement is the second lowest (5.62 ± 5.1) of the 3 control groups (with those who have neither mobility impairment nor NB having the highest average endorsement rate [7.0 ± 4.4]). A final aspect of validity was explored by studying whether respondents tended to attribute the symptoms to a UTI. This feature of the instrument was challenging to conceptualize, not only because the frame of reference for endorsement was the previous year, but also because attribution, by the patient, of a symptom to a UTI could arise from a wide variety of ultimately unverifiable rationales.
Because it is, and was, impossible to verify whether any experience was actually a UTI, we instead present results based on respondents' assertions that the symptom was never associated with a UTI. This would therefore exclude all the above possible associations with, or attributions to, a UTI. Therefore, Table 7 presents the proportions of respondents, collapsed over our national patients-with-NB samples (n = 581) and control (n = 160) groups. In each case, the proportions of those who endorsed each item and never attributed it to a UTI are given.
Table 7
Proportions of Respondents Endorsing a USQNB-IC Item and Not Attributing It to a UTI.
Taken together, these results suggest that the USQNB-IC possesses important validity evidence according to the COSMIN criteria. Specifically, the results present several lines and sources of evidence of face, content, criterion, convergent, and divergent validity for these items; reliability evidence (Table 4) also arises from the results considered all together.
Methods: SA2 SMP-Pro Development
Concurrent with the nationwide validation studies of the USQNB-IC (SA1), we developed the SMP-Pro, an algorithm to be used by study participants during the intervention phase to determine what to do when having urinary symptoms (increase fluid intake and catheterizations, start LGG treatment, or seek medical attention). Development of the SMP-Pro was an iterative participatory design process that first involved patient focus groups and then iterative collaboration with consumer experts and study clinicians. Once a prototype of the SMP-Pro was developed (first interviews, then the iterative collaboration), we worked with a diverse sample of individuals with SB and SCI to develop use cases and scenarios of use to further inform the protocol. A representative study sample to test the SMP-Pro was purposively recruited, in conjunction with national piloting of the beta USQNB-IC, to include adults and children of both sexes as well as persons who reuse catheters and those who do not to ensure that the SMP-Pro tool could be used by all study participants. We selected these participants to be representative of the anticipated participants in the intervention portion of the study.
Six participants were recruited for each of the following 4 groups (24 participants total) to participate in semistructured interviews: (1) those with NB who use IC; (2) caregivers of someone with NB who uses IC; (3) clinicians who treat those with NB; and (4) those who either have a history of UTIs or care for people with NB plus UTI, or clinicians working with people with NB plus UTI. During the interview they were asked to recall the onset and progression of symptoms in their most recently diagnosed UTI. The interviewer then asked the study participants to mentally step through the experience of symptoms and UTI (1) to confirm that the use of the prototype SMP-Pro could be triggered by the symptoms endorsed on the USQNB-IC and (2) to elucidate the mechanics of implementing the protocol across the diverse group of individuals with NB due to SB or SCI to determine how/when patients should implement the SMP-Pro and to address any obstacles that might occur. Interview data were managed, coded, and thematically analyzed using NVivo 1059 qualitative software. The most important discovery of activation testing between the symptoms on the USQNB-IC that trigger the use of the SMP-Pro was that patients who use closed catheter systems do not experience the symptom of malodorous urine and that malodorous and cloudy urine typically occur together. As a result, the interdisciplinary team refined and expanded the SMP-Pro to accommodate participant experience augmented by use cases and scenarios of use. Additionally, the activation testing informed the sequencing of the protocol-guided instillations to accommodate real-life use.
Once the SMP-Pro was finalized, we developed instructional materials, both paper and video, explaining the use of the USQNB-IC and SMP-Pro. Consumer experts were integrally involved in the development of these materials, as described previously.
Changes to the Original Study Protocol for SA2
We added an additional 3 months to the follow-up phase, as a safety consideration. This was a requirement of the Investigational New Drug (IND) application by the FDA (see Appendix D).
We changed our recruitment balance of adults and children enrolled in the study. We had initially proposed an equal distribution of adults and children; however, we experienced numerous challenges in the recruitment of children, specifically due to a lack of proficiency in understanding and being able to communicate in English (a preponderance of potential participants were primary Spanish speakers), challenges in agreement of both parents and the child to participate, and a perception by children and their parents of undue burden and duration of the study. Therefore, after consultation with our PCORI project officer, we altered our recruitment such that we recruited additional adults to compensate for the underrecruitment of children, while still reaching our total enrollment goals.
All changes were approved by the MedStar IRB and, when required, by PCORI.
Results: SA2 SMP-Pro Development
The resulting SMP-Pro is depicted in Figure 1.
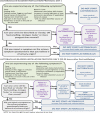
Figure 1
Self-management Protocol Using Probiotics.
Methods: SA2 SMP-Pro Pilot Trial
Before starting the pilot trial, we completed a small safety study requested by the FDA. Results can be found in Appendix D. The pilot trial was conducted using a national sample with study participants from 23 states. This nationwide sample was planned to ensure that we obtained a diverse sample of participants. Consent was obtained by the study coordinator or research assistant and overseen by the principal investigator. All project staff completed Research Ethics and Compliance Training through the Collaborative Institutional Training Initiative61 and had passed all MedStar HIPAA certifications.
Inclusion criteria for study participants with SCI included the following:
- Age ≥18 years
- SCI at least 1 year in duration
- NB, as determined by the attending physician
- Using IC for bladder management
- History of ≥2 UTIs in the past year
- Community dwelling
Inclusion criteria for study participants with SB included the following:
- Age ≥6 years
- NB, as determined by the attending physician
- Use of IC for bladder management
- History of ≥2 UTIs in the past year
- Community dwelling
Inclusion criteria for study participants with MS included the following:
- Age ≥18 years
- MS at least 1 year in duration
- NB, as determined by the attending physician
- Using IC for bladder management
- History of ≥2 UTIs in the past year
- Community dwelling
Exclusion criteria included the following:
- Known GU pathology beyond NB (ie, vesicoureteral reflux [VUR], bladder or kidney stones, etc)
- Use of prophylactic antibiotics
- Instillation of intravesical agents to reduce UTI (ie, gentamycin)
- Psychologic or psychiatric conditions influencing the ability to follow instructions
- Participation in another study in which results would be confounded
- Pregnant or breastfeeding
- History of acquired genetic immunodeficiencies or active or chronic serious infections
- Cancer or autoimmune disorders
- Serious allergy to any part in the live bacterial combination
- Change in their SCI or SB in the past 2 weeks
- History of sensitivity or allergy to ampicillin or tetracycline
All persons with VUR were excluded for safety reasons because this was the first in-human trial using intravesical LGG. If participants were taking antibiotics at the time of enrollment, a washout of 2 weeks antibiotic free was conducted to ensure that no participants were taking antibotics when they started the study.
Participants initially completed the USQNB-IC weekly (baseline phase) for 6 months to establish a baseline level of urinary symptoms and AEs (related or unrelated to NB). After 6 months of observation to establish a baseline urinary symptom experience, study participants received face-to-face training on how and when to administer the LGG probiotic, as guided by the SMP-Pro, including preparation and intravesical instillation of the LGG probiotic with supplies provided (catheters, LGG probiotic, lubricant, gloves, alcohol swabs, catheter tip syringe, saline, and urine cups). This was accomplished via a face-to-face (electronically or in-person) tutorial with a consumer expert. The SMP-Pro and LGG bladder instillation instructions (including a step-by-step video) were provided in hardcopy and by email at the time of the study participant/consumer expert training. For the intravesicular LGG instillation, study participants were instructed to mix the contents of 1 LGG capsule with 10 billion colony-forming units (brand: Culturelle Probiotic) into 45 mL of sterile 0.9% saline62,63 (the amount of sterile saline differed for pediatric study participants, as they used an age/size-adjusted instilled volume). After mixing, study participants were instructed to draw up 45 mL of the liquid LGG mixture into a 60-mL catheter tip syringe and instill via the intermittent catheter after emptying the bladder by catheterization. Study participants received 10 LGG capsules at a time. All participants were instructed to notify the study coordinator when they had 2 capsules remaining and at that time he or she would then dispense additional LGG. At the end of the intervention, any unused capsules were returned to the study coordinator and counted.
During this 6-month intervention phase, if/when urinary symptoms occurred, study participants were instructed to follow the SMP-Pro to determine whether to initiate intravesical LGG instillation, increase water intake, or be evaluated by a health care professional. The SMP-Pro also directed them to discontinue LGG probiotic instillation or be evaluated by a clinician if symptoms remitted, persisted, or worsened. If study participants were directed by the SMP-Pro to be evaluated by a clinician or felt the need for medical evaluation, they were advised to obtain care as they typically would by a health care provider. Study participants were supplied with letters to be brought to their health care provider notifying them of the study and requesting sharing of urinalysis and urine culture results with the research team. A “verified UTI” included those that resulted in antibiotic treatment by a health care professional (see Figure 1).
After completion of the 6-month participant-initiated SMP-Pro intervention phase, study participants monitored symptoms weekly using the USQNB-IC for a 6-month washout phase (just as in the initial 6-month baseline phase).
To assess safety, per FDA requirements, we followed AE and SAE reporting according to the FDA IND safety reporting requirements outlined in 21 CFR 312.32.64 An AE (21 CFR 312.32[a]) was defined as “any untoward medical occurrence whether or not it was associated with the use of the study drug and whether or not it was considered drug related.” An SAE (21 CFR 312.32[a]) was identified when any of the following occurred: death, a life-threatening AE, inpatient hospitalization (or prolongation of existing hospitalization), or a “persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions.” A data and safety monitoring board (DSMB) was established and tasked with determining the relatedness of the SAEs to the intervention using the following criteria: “definitely unrelated,” “unlikely to be related” or “possibly,” “probably,” and “definitely” related. Additionally, we asked participants to describe whether or not they considered an AE or SAE to be related to the study drug.
All AEs and SAEs were recorded by participant report and provided to the DSMB at 6-month intervals. Only SAEs that occurred at any time after LGG instillation were reviewed by the DSMB for independent determination of relatedness to the intervention. For all SAEs classified as GU infections that occurred at any time after LGG instillation, urinalysis and urine culture results were obtained and provided to the DSMB. If the participant experienced an AE or SAE but had not instilled LGG, then the event was classified by the study clinician as “definitely unrelated” to the intervention.
To assess tolerability, we created a 4-item survey that we administered at the end of each phase of the study (every 6 months). Participants used a Likert scale to rate their satisfaction with changes in the impact, frequency, and severity of their symptoms in the previous 6 months with 3 questions. As a participant-reported indicator of the tolerability of the instilled LGG, we used their answers to the fourth item, “While thinking only about the preceding 6-month time period: Can you estimate, using a scale from 0 to 100%, whether or not you would seek out this intervention and pay for it yourself if insurance did not pay for it?” Participants indicated their answer by moving a “slider” to 1 of 3 positions: 0% = Would absolutely never do this; 50% = Might do this; 100% = Would absolutely do this. For our tolerability analyses, we sought to understand how and whether the likelihood to seek and pay for this intervention might be associated with any AEs experienced by our participants. “Tolerability” of the intervention was thus defined as statistical independence of the likelihood to seek and pay for this intervention and the individual's total AE experience throughout the trial. Statistical independence would be observed as a lack of association between number of AEs (total) and doses of LGG, and the number of AEs (total) and subjective ratings of willingness to seek/pay for the intervention. Tolerability was only estimated for those who instilled at least once.
To assess urinary symptom burden (primary outcome), we estimated burden for each of 3 symptom group types identified for the USQNB-IC.65 The classification of the 29 USQNB-IC symptoms and denominators for each type of burden was as follows:
To summarize burden in each of these symptom types over multiple weeks, we tallied the experience of each symptom type (total number of endorsed symptoms of each type) for the entire 6-month phase, tailoring this computation to the number of weekly USQNB-ICs that a given participant completed during that phase (maximum = 24 weeks). The overall experience of that burden type for this phase of the study was divided by a denominator formed by the product of (number of possible symptoms in that type) and (number of weeks of participation in that phase).
Change in burden of a given symptom type (eg, clinically actionable or A-type) between phase 2 (intervention) (P2_A) and phase 3 (washout) (P3_A) was estimated as the difference in burden (subtraction) with a correction for the baseline level. This corrected difference was our “baseline-adjusted” estimated change: (P2_A – P3_A)/P2_A where “baseline adjusted” was defined as correcting for the summary in the earlier phase (dividing by P2_A in this example). These change scores could then be compared within-person using a 1-group t test against the test value of 0 (ie, no change = 0). We also defined a “response” to be a “baseline-adjusted” estimated change of at least 10% for that symptom type. This response variable (yes/no) was estimated for each symptom type (A, B1, B2, and C). A “clinically meaningful effect” was defined to be a 10% reduction in symptom burden in P3 relative to P2 for those who did instill at least once in P2. Although this 10% threshold has not previously been reported, it was determined a priori by our research team of clinicians and consumer experts as representative of clinically meaningful effect in symptom reduction based on their collective experience and expertise.
Based on our subject matter expert characterization of the C-type symptoms as noninformative for urinary symptom management,65 we hypothesized no association with instillation/no response in the C-type symptoms over time. Our national validation sample58 suggested that the intervention “trigger” symptoms (cloudier or fouler-smelling urine) were associated with a UTI at least some of the time, so we hypothesized that symptoms in types A, B1, and B2 would be improved if the intervention was used and was effective.
We did not randomly assign participants to instill or not; all those who were enrolled were eligible to instill (following the protocol; see Figure 1). We hypothesized that any changes in burden that noninstillers experienced would be similar between baseline and intervention (P1 vs P2) and between intervention and washout (P2 vs P3), while we hypothesized that those who did instill at least once during P2 might experience increased, or unchanged but remaining high, burden between baseline and intervention (P1 vs P2), and then decreased burden between intervention and washout (P2 vs P3) if the intervention exerts a lasting effect.
Instillers were defined as those who instilled at least once during their time in the 6-month intervention phase (whether or not they dropped out before completing this phase). Participants who instilled at least once (instillers) and those who never experienced the 2 trigger symptoms and did not instill (noninstillers) cannot be compared statistically beyond descriptions of background characteristics. Individuals who ended up in these groups had qualitatively different symptom experiences; they were not randomly assigned to be an instiller or noninstiller. We therefore carried out within-group 1-sample t tests on adjusted change in burden for instillers and noninstillers separately. Conclusions about the effectiveness of the intervention are based on within-participant (burden estimates) and within-group (instillers-only) analyses. These effects for instillers are contextualized against the noninstiller experience.
Urinary symptom burden was estimated for each of 3 symptom group types identified for the USQNB-IC. The classification of the 29 USQNB-IC symptoms, and denominators for each type of burden, is shown in Table 8.
Table 8
USQNB-IC Symptoms.
To summarize burden in each of these 3 symptom types over multiple weeks, the denominator (number of symptoms in that type) was multiplied by the number of times the survey was completed (number of weeks of participation). To estimate change in burden, we computed the simple difference between burden of a given type in P1 and P2. Then, we subtracted the relevant P2 value from the relevant P1 value, and divided by the earlier phase's value to get a “baseline-adjusted” estimate of change: (P1 – P2)/P1, for each symptom type.
Adults and children were analyzed together (their burden estimates were all computed the same way) because there were only 5 children who instilled and 2 who never did—insufficient numbers to support valid inferences. We first examined adjusted change in burden between the baseline and intervention phases (P1 and P2), and then examined change between the intervention and washout phases (P2 and P3). In all analyses, α was set at .05; we did not seek “statistically significant” results in these t tests, but rather evidence of whether those who did instill in P2 would have reductions in symptom burden in the washout phase. Therefore, we did not correct for multiple comparisons. Instillers and noninstillers had qualitatively different experiences and were therefore never compared. Missing data were not imputed.
Statistical Analyses
All data were collected using Research Electronic Data Capture (REDCap) and exported as a .csv file to SPSS v. 25 (IBM, Inc) for analysis. We used 1-sample, 2-tailed t tests to test change in burden of symptom types A, B1, B2, and C between P2 and P3. Analyses were carried out separately for those who never instilled during the phase where this was possible (P2) and those who did instill at least once. Individualized change in burden was computed such that negative values would indicate increases in burden and positive values would indicate decreases in burden. If zero symptoms were observed in 1 phase, and if nonzero was observed in the other phase, the baseline corrected change was “100%”—either 100% increased or 100% decreased.
Adults and children were analyzed together (their burden estimates were all computed the same way; see Tractenberg et al65 for scoring details) because there were only 5 children who instilled and 2 who never did, representing insufficient numbers to support valid inferences. In all analyses, α was set at .05; we did not seek “statistically significant” results in these t tests, but rather evidence of whether those who did instill in P2 would have reductions in symptom burdens of types A, B1, and B2 (but not C) in the washout phase. We applied the Holm correction52 for 4 multiple comparisons (1 for each t test of change in burden on 4 symptom types). The primary outcomes were changes in burden between the intervention and washout phases (P2 and P3). Uncorrected exploratory analyses of changes in burden between the baseline and intervention phases (P1 and P2) were also planned. Missing data was not imputed.
Data Collection
All data were collected and managed in the REDCap system hosted by the Biostatistics and Informatics Core of the Children's Research Institute's Center for Clinical and Community Research at the Children's National Medical Center (https://cri-datacap.org/). The REDCap system is a secure, web-based application designed to support data capture for research studies and provides (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Weekly review of data entry occurred every Tuesday morning (between the date the first participant was enrolled and the date the last participant completed week 72). Team members checked the scheduled-events database to see which survey invitations and reminders had not been responded to and contacted the appropriate participants by telephone and/or email to encourage survey completion. Staff outreach efforts were entered in the “survey reminder” form in REDCap.
Evaluation of AEs occurred during the weekly review. Team members also checked the database to determine which participants submitted survey responses that were indicative of AEs related to study participation and medical care–seeking behavior. If a survey response indicated that a participant thought he or she was having an AE related to study participation or indicated that a participant was admitted to the hospital or sought medical care, the study coordinator contacted the participant to obtain information about symptoms or AEs, or medical care presentation, diagnosis, and treatment to determine if the event should be considered an AE. Information from these evaluations was entered into REDCap and reviewed by the principal investigator.
Team members also conducted teaching efforts as study participants prepared to begin each new 6-month period, informing participants whether the period would include urinary symptom surveillance by the USQNB-IC, or symptom surveillance and LGG instillation per the SMP-Pro.
Participant Withdrawal
For each withdrawal, the reason was entered into REDCap and into a password-protected MS Excel sheet. Before being determined lost to follow-up, the participant was contacted multiple times per week until starting a new phase in the study. If not reached at that time, the participant was classified as lost to follow-up.
Figure 2 is a CONSORT diagram of screened, enrolled, included, and withdrawn participants.
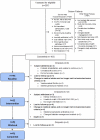
Figure 2
CONSORT Participant Flow Diagram.
Results: SA2 SMP-Pro Pilot Trial
Table 9 summarizes the demographic characteristics of study participants.
Table 9
Demographics of Study Participants in SMP-Pro LGG Prospective Intervention Trial.
Intervention Use
The 59 of 88 adults (67%) who entered P2 self-administered between 1 and 41 instillations, for a total of 324 (90.8%) instillations. Five of the 7 children (71%) instilled LGG, administering 3 to 9 doses per participant, for a total of 33 (9.2%) LGG instillations.
Safety Assessment
There were 59 AEs plus SAEs (13 AEs and 46 SAEs) that occurred over the 18-month period. All (100%) of the 13 AEs occurred in 11 adult participants; no AEs were experienced by child participants. Of the SAEs, 95.7% (44 of 46) occurred in 27 adults and 4.3% (2 of 46) were experienced by 2 child participants. Nearly half (26 of 59 [44%]) of all AEs plus SAEs were classified as GU infections. The 2 SAEs that occurred in children were in different participants, and both occurred before the instillation phase; 4 of 13 AEs and 22 of 46 SAEs in adults occurred before the instillation phase.66
Relatedness of AEs plus SAEs to the intervention
Of the 13 AEs, 6 (46.2%) were experienced by adults who had instilled LGG at any time before the AE; all 6 of these AEs were rated as moderate in their severity (per FDA definition),67 and included irritation after instilling (1 AE), UTI (1 AE), emotional discomfort due to a UTI (1 AE), UTI and kidney infection (2 AEs), and migraine (1 AE). Two of these AEs, irritation instilling LGG (1 AE) and UTI/ kidney infection (1 AE), were considered by the participants to have been “caused by LGG,” and a third (migraine) was considered by the participant as “maybe caused by LGG.”
Of the 46 SAEs, 22 (47.8%) occurred during P1 (baseline). The remainder (24, or 52.2%) occurred during the ensuing 12 months (P2 and P3 combined, at any time after LGG could have been instilled). Of all SAEs, 23.9% (11) occurred at some point after the participant indicated they had used LGG. Of these 11 SAEs, 4 were considered by the DSMB to be “unlikely to be related” to the study drug (ie, LGG) and included bladder infection (1 SAE), prostate infection (1 SAE), severe kidney infection (1 SAE), and coughing and urinary symptoms (1 SAE), while 7 of 11 were definitely unrelated to the study drug. None were determined by the DSMB to be “definitely,” “probably,” or “possibly” related to the study drug.66 Table 10 provides counts of SAEs and AEs.
Table 10
Counts of SAEs and AEs.
Variability of AEs and SAEs by Study Phase
For the 27 participants who entered the intervention phase (P2) but never instilled, an average of 0.51 AEs plus SAEs occurred in P1 (baseline) and 0.074 occurred on average during the remaining 12 months of the study. These participants experienced an average of 0.30 GU AEs (GU plus GU infections) in P1 and no GU AEs in P2 or P3 (washout). For the 64 who instilled at least once, during the baseline phase they experienced an average of 0.36 AEs plus SAEs, including an average of 0.16 GU AEs. Over the next 12 months, these 64 instillers experienced an average of 0.28 AEs plus SAEs, including an average of 0.16 GU AEs. The paired t tests comparing experience (AE plus SAE or GU AE) during the baseline 6 months with the 12 months of P2 and P3 were not statistically significant for total AE count 9t[63] = 0.76) or for those AEs designated GU or GU infectious (t[63] = 0; both P > .40). Occurrences of GU AEs plus SAEs decreased over the 18 months of the study, but for instillers, the correlation between total AEs during the final 12 months and total doses instilled was significant (r = 0.291, P = .02). One individual instilled 41 times, while the next highest dose count was 14. Without this outlier, the correlation coefficient dropped (r = 0.255) and the association marginally failed to reach significance (P = .055). With and without this extreme dose value, the association between dose and total AE count was not strong (squaring the correlation coefficients shows dose and total AE count share 6.5% to 8.5% of variance). In summary, instillers had the same rate of GU AEs plus SAEs in the first 6 months as in the last 12 months, but their overall AE-plus-SAE rate decreased slightly from 0.36 (first 6 months) to 0.28 (last 12 months) events per person, on average.
Figure 3 shows the distribution of doses according to the total number of AEs and SAEs experienced over the 18-month study for instillers. The participant instilling 41 times experienced 2 AEs; both occurred after at least 1 instillation.66

Figure 3
Distribution of Doses and AEs and SAEs Experienced Over the 18-Month Study for Instillers.
Rate of AEs and SAEs by Exposure to Intervention
We further described all AEs plus SAEs by person-time in the study to better summarize participant experience (total unique AE plus SAEs divided by the total number of weeks participants filled in the survey, multiplied by 52 weeks). The events per person-year were computed separately for instillers and noninstillers. For instillers, preinstallation rate was based on the number of weeks from consent to the week before first instillation, while for noninstillers, preinstallation rate was the number of weeks they participated each period. Instillers experienced 0.084 events per person-year before any installation. During the installation phase (defined per individual at first instillation and ending after their 48th week in the study), instillers experienced 0.43 events per person-year. During postinstillation (defined per individual from the week after their last instillation through their 72nd week of the study), the rate of AE plus SAE was 0.178 events per person-year. For noninstillers, the event rates were 1.35, 0.906, and 0.206 events per person-year in the first, second, and third phases, respectively. Since the groups are not comparable, no inferences were planned or done.66
Tolerability Assessment
Of the 64 instillers, only 55 provided tolerability ratings for these analyses; for these raters, total AE plus SAE experience was classified as 0 (n = 37 [67.3%]), 1 (n = 11 [20.0%]), or 2 to 6 (n = 7 [12.7%]) AEs plus SAEs. The average ratings of a person's likelihood to seek and pay for the intervention, rated at the ends of P2 (intervention) and P3 (washout) did not differ significantly across the AE plus SAE experience groups [F(2,47) = 2.4, P = .07]. However, the average tolerability ratings of those with the greatest numbers of AEs plus SAEs (2-6) were >20 points below the averages of the other 2 AE plus SAE count groups, which were nearly identical (0 AEs plus SAEs = 63.9; 1 AE plus SAE = 64.1). Figure 4 shows that the 2 lowest likelihood ratings, as well as one of the highest, were recorded for those with 2 to 6 total AEs plus SAEs over the 18-month study. The next lowest, and 4 highest, were among those with 0 AEs plus SAEs. Figure 4 suggests that total AEs plus SAEs was unrelated to the total number of doses of LGG instilled. The distributions of ratings for likelihood of seeking/paying for the intervention (x-axis) were very wide for all AE plus SAE experience groups, including those with no AEs plus SAEs (stars in Figure 4).66

Figure 4
Likelihood of Seeking/Paying for Intravesical LGG.
Effectiveness
Effectiveness of the SMP-Pro LGG instillation was assessed by measuring symptom burden (from the USQNB-IC). Because there were so few children (and all burden estimates were computed in the same way), we combined all completers in our analyses. We separated the summaries of the descriptive characteristics by those who instilled LGG (per the SMP-Pro) at any time (instillers) and those who did not use LGG at any time (noninstillers).
Noninstillers (n = 27) were aged 41.5 years on average (SD, 16.3 years); 67% were male. Of these patients, 81.5% (n = 22) were adults and 1 child with SCI, and 11.1% (n = 3) were adults and 1 child with SB. Instillers (n = 64), defined as those who instilled at least once during their time in the 6-month intervention phase (whether or not they dropped out before completing this phase), were aged 40.8 years on average (SD, 16.2 years); 56.2% of these participants were male, and they ranged in age from 8 to 74 years old at the start of this 18-month-long study (see Table 11).
Table 11
Symptom Burden During 6-Month Intervention Phase.
Although noninstillers tended to be less symptomatic—as expected given that they did not ever instill—there were no statistically significant differences between instillers and noninstillers in their weekly symptomaticity (endorsing at least 1 symptom in 37% of their weeks in study vs. 47.6% of their time in study for instillers). Noninstillers averaged fewer episodes taking antibiotics (6.85 times vs. 9.7 times for instillers) and sought medical attention roughly the same amount of time (5.93 times for noninstillers vs 5.97 times for instillers; all P > .17). On average, P2 instillers met instillation protocol criteria 3.83 times, with a range of 1 to 20 times during this 24-week (6-month) period. In those 3.83 weeks, an average of 5.6 doses of LGG were instilled (translating to nearly 2 doses per instillation period).
Urinary symptom burden was estimated for each of 3 symptom group types identified for the USQNB-IC. To summarize burden in each of these 3 symptom types over multiple weeks, the denominator (number of symptoms in that type) was multiplied by the number of times the survey was completed (number of weeks of participation). As described previously, to estimate change in burden, we computed the simple difference between burden of a given type in P1 and P2 by subtracting later from earlier burden and dividing by the earlier phase's value to get a “baseline-adjusted” estimated change: (P1 – P2)/P1 for each symptom type.
We carried out 1-sample t tests on adjusted change in symptom burden for instillers and noninstillers separately. When we stratified USQNB-IC symptoms into groupings such that A = actionable symptoms; B1 = bladder function symptoms; B2 = bladder/urine quality symptoms; and C = constitutional symptoms, we found significantly more B2 symptoms in P2 vs P1, after adjusting for P1 experience, in those who instilled (t [63] = −2.47, P = .016) (see Table 11).
When we compared adjusted change in burden between P2 (intervention) and P3 (washout), there were no significant differences in symptom burden of any type for those who never instilled (all P > .17). Some types increased, some types decreased, but none changed significantly between P2 and P3 after controlling for P2 burden levels. Instillers experienced significantly fewer USQNB-IC A-type symptoms (actionable) and B2 (bladder/urine quality) symptoms in P3 compared with P2 after adjusting for P2 experience (A: t [44] = 4.63, P < .001; B2: t [56] = 2.67, P = .01) (see Table 12).
Table 12
Adjusted Changes in Burden by Symptom Type for Instillers and Noninstillers.
Methods: SA3 Impact of Self-management With LGG on Bladder Inflammation and Urine Ecosystem
Participants
We recruited 28 participants living in the Washington, DC, metropolitan area from the sample in SA2 to participate in SA3. We collected urine at multiple time points (baseline, healthy urine sample monthly during baseline and intervention, before and after SMP-Pro–guided LGG instillation during intervention, and 1 sample during the follow-up phase) to estimate the strength of the associations between successful implementation of the SMP-Pro and urinary symptoms, bladder inflammation, and the urine microbiome.
Urine Collection
Asymptomatic urine samples were collected in P1 and monthly during P1 and P2. During P2, urine was collected before an instillation occurred and within 48 hours after the last instillation. Last, a 3-month follow-up urine collection occurred during P3.
A 50- to 100-mL urine sample was collected from study participants at each urine collection for (1) urinalysis and culture and (2) urine DNA sequencing. Urine was collected from a new, unused intermittent catheter. Collected urine was immediately placed at 4 °C. Within 6 hours, samples were taken to our partner institution, Children's National Medical Center (CNMC) for sequencing preparation. At CNMC, urine samples were centrifuged at 4 °C, 5000g for 20 minutes. The supernatant was aliquoted in 2-mL cryotubes and frozen at −20 °C. Then, 10 mL of phosphate-buffered saline (PBS) was added to the pellet with the remaining supernatant and centrifuged (5000g) at 4 °C for 20 minutes. The pellets and aspirated PBS wash solution were stored at −20 °C. Upon study completion, pellets were transferred to the Genomics and Epigenomics Shared Resource Laboratory at Georgetown University Medical Center for DNA isolation and sequencing.
Urinalyses were assessed using standard clinical microbiology semiquantitative chemical testing using commercial disposable test strips for glucose, bilirubin, ketone, specific gravity, blood pH, protein, urobilinogen, nitrite, and LE. After centrifuging for 5 minutes, microscopic examination for and quantification of WBCs, red blood cells (RBCs), epithelial cells, yeast, bacteria, Trichomonas vaginalis, sperm cells, mucous filaments, and crystals was performed using standard microbial techniques.
Standard urine culture microbiology was performed to assess bacterial load. A measured amount was inoculated to each of the appropriate media. A calibrated loop designed to deliver a known volume (0.01 mL per loopful) of urine was used. The sample was mixed thoroughly, and the top of the container removed. A calibrated wire-inoculating loop was flamed and allowed to cool without contact with any surface. The sterile loop was inserted into the urine sample vertically, allowing urine to adhere to the loop. The loopful of urine was inoculated over MacConkey agar plates using standard methods. Similarly, a second loopful was collected and inoculated on a blood agar plate. The plates were incubated aerobically at 35 °C to 37 °C for at least 24 hours. The colonies and colony-forming units were counted by multiplying by 100 (because a 0.01-mL loop was used).
Next, 16S sequencing was performed to more fully identify the bacterial community in the urine at various time points. For sequencing analyses, urinary bacteria were pelleted using low-speed centrifugation, and the resulting pellets were washed with PBS (10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM NaCl, and 2.7 mM KCl) and stored at −80 °C. Depending on the size of the pelleted material, genomic DNA was isolated either with the DNeasy Kit (Qiagen), using the manufacturer's protocol for gram-negative bacteria, or with the QIAamp DNA Micro Kit (Qiagen), using the manufacturer's protocol for DNA isolation from urine. Purified DNA was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific). Fractions of human and bacterial DNA in each sample were determined using Femto Human and Femto Bacterial DNA quantification kits (Zymo Research), according to the manufacturer's instructions.
Per standard practice, V4 regions of 16S ribosomal RNA (rRNA) genes were amplified using primers 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA-3′ and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTWTCTAAT-3′ and the following reagent concentrations: 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 200 μM of each deoxynucleoside triphosphate, 2 μM of each primer, 1% glycerol, 0.3 U AccuPrime Taq polymerase (Thermo Fisher Scientific) and 25 ng of template DNA in a 20-μL total volume. Amplification conditions were 2 minutes at 95 °C initial denaturation, followed by 30 cycles of 20 seconds of denaturation at 95 °C; 15 seconds of annealing at 55 °C and 5 minutes of extension at 72 °C; and a 5-minute final extension at 72 °C. Amplification products were purified with the AMPure XP system (Beckman Coulter) and their size was verified with the DNA 1000 kit (Agilent). Indexing and pooling of amplification products was carried out according to Illumina's 16S metagenomic sequencing library preparation protocol. The resulting library was sequenced using Illumina MySeq reagent kit v2 (500 cycles). Sequenced rRNA genes were analyzed and classified using PathoScope. Single-value summaries of the species diversity were computed for each sample, including the Shannon diversity index and the abundance-based coverage estimator (ACE), which we added as a descriptor post hoc after finding evidence that our samples required an estimator that is more robust to rare species than the Shannon index.
Changes to the Original Study Protocol for SA3
Due to the previously noted challenges in recruiting children for SA2 and the resulting change in the adult:child participant distribution ratio, we reduced our enrollment of study participants for SA3 from the proposed 50 to 28. We were able to compensate for fewer participants in the urine sample subaim by collecting additional urine samples from these participants. Although we had initially proposed urine collections at 3 time points (P1 [baseline], end of P2 (intervention) using the SMP-Pro, and end of P3 [follow-up during washout]), with fewer (and adult only) participants providing urine samples, we instead collected urine at multiple time points (up to 18 unique times): (1) up to monthly for the first 6 months of the study when participants did not have any urinary symptoms; (2) up to 2 times when participants experienced urinary symptoms (before LGG instillation); (3) follow-up after either LGG instillation or antibiotics; and (4) three months into P3.
All changes were approved by the Medstar IRB and, when required, by PCORI.
Results: SA3 Impact of Self-management With LGG on Bladder Inflammation and Urine Ecosystem
We defined “successful implementation” a priori as instillation that resulted in at least a 10% decrease in total symptoms. Although we were able to determine who had “successfully” implemented the SMP-Pro and to analyze associations between implementation and urinary symptoms, there were insufficient numbers of individuals with both pre- and postinstillation urine samples (n = 11) to formally test hypotheses about, or estimate the strengths of, associations between response to instillation and changes in the markers of bladder inflammation (ie, nitrites, LE, and WBC counts). Instead, our analyses focused on describing the 64 participants who used the SMP-Pro at least once during the 6-month intervention phase. These individuals were stratified (post hoc) into 2 groups: those who responded to SMP-Pro guided LGG instillation and those who did not. We had defined “response” as the observation of a ≥10% decrease in the average number of USQNB-IC symptoms endorsed (summarized per 6-month period) so that each of the individuals in this subsample was characterized as either a responder (≥10% decrease) or a nonresponder (0%-9.99% decrease). Thus, considering the set of USQNB-ICs that any given participant filled out during the intervention phase of the study, of the 64 participants who instilled LGG at least once during this phase, 49 (76.6%) experienced at least a 10% decrease (improvement) in total average symptoms, while 15 experienced no change (0% decrease, 5 cases), improvement (0.10%-9.99%, 1 case), or worsening (9 cases) (see Figure 5).
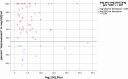
Figure 5
Change in USQNB-IC Endorsed Items Pre- and Postinstillation.
Figure 5 shows that the majority of individuals who instilled also met our definition of “responder.” There were no significant differences in the prior-to-instillation scores of those instillers who did, vs those who did not, qualify as a “responder” (t [62] = 0.54, P = .50).
Urine Samples
We obtained the quantification of WBCs, RBCs, epithelial cells, yeast, bacteria, Trichomonas vaginalis, sperm cells, mucous filaments, and crystals using standard microbial techniques. Urine samples were collected from local participants (those located in close proximity to the study center), but not all urine samples were given by individuals who used the SMP-Pro. Urine samples were collected when participants were either asymptomatic, symptomatic both before and after LGG instillation, and at a 12-week follow-up. Only local participants could provide urine samples due to the nature of having to meet in-person to collect the urine sample. Further, only local participants who experienced urinary symptoms could provide “symptomatic” urine samples. Nonsymptomatic samples were collected monthly if no symptoms occurred to initiate an instillation. We collected 141 samples. Some samples were collected before an instillation, but because antibiotics started before a postinstillation sample could be collected, we did not collect postinstillation samples. We summarize the urinalysis results briefly below, and then focus on the inflammatory markers.
Inflammatory Markers
Across the 141 samples for which urinalysis and urine culture were completed, 77 (54.6%) were negative for nitrites. LE was negative in 38.3% and trace in 7.1% of the samples; 23.4% were ≥1 and 30.5% were ≥2 for LE. In terms of WBC counts (high-power field [hpf]), samples were distributed across 0 WBCs (12.1%), 0 to 5 WBCs (43.3%), and 6 to 10 WBCs (14.2%); the remaining 29.8% of samples had WBC counts of 10 to 20 (17.7%), 20 to 40 (4.3%), and 40 to 60 (3.5%). Five samples (3.4%) had ≥60. The vast majority of RBC/hpf values in these samples were 0 (63.8%) or 0 to 2 (28.4%). The remaining 10 cases (7.1%) were 0 to 5 (0.7%), 3 to 10 (3.5%), 10 to 20 (1.4%), 40 to 60 (0.7%), or ≥60 (0.7%). Epithelial cells, yeast, bacteria, Trichomonas vaginalis, sperm cells, mucous filaments, and crystals were not reported in our standard urinalysis or culture results, and because these are not typically used for diagnosis or other clinical determinations, we do not report them.
The estimated strengths of association between key markers of inflammation and total symptom count were not high. Associations were estimated using Spearman (nonparametric) estimation. Total symptom count correlated with nitrite levels (ρ = 0.287; P = .001), suggesting that while statistically significantly different from 0, only ρ2 = 8% of variance in nitrite levels and total USQNB-IC scores are shared, a marginal association. Correlations between total symptoms and LE (ρ = 0.110, P = .22) or WBC counts (ρ = 0.126, P = .16) were not significant.
Of the 141 samples, 20 samples from 13 individuals corresponded to instillation of LGG, with a urine collection performed before and after the instillation occurred. There were no significant associations (all P > .49) between any inflammatory marker and total symptom count when the samples were analyzed separately depending on whether (n = 20 samples) or not (n = 32 samples) an instillation had occurred. The majority of samples were collected during study weeks outside P2, and only 11 individuals contributed samples before and after the instillation. During P1 and P3, the association between nitrite level and total symptom count was ρ = 0.30 (P = .009). As noted, we defined “successful implementation of the SMP-Pro” as one that led to a reduction of at least 10% in total symptoms; this was estimated based on the individual's average experience throughout P2. However, it is not possible to draw clinical inferences about the association between response (successful implementation) and urine sample results, because of the combination of complexities from (1) estimating “successful implementation” based on observations over roughly 6 months' worth of weekly data and (2) having collected urine after the intervention (which only resulted in 11 pre- and postinstillation samples); this yielded a data set too small for formal inferential analyses.
The strength of the association between bacterial composition, as determined by metagenomics, was first assessed using the Shannon diversity index. The Shannon index is a single-value summary used to describe the diversity (number of different types or species) of microorganisms in a single sample. Only a subset (n = 121) of the 141 urine samples yielded an analyzable sample for the microbiome analysis (due to the amount of bacterial DNA present). Because we had multiple samples per person, but not all the urine samples were taken when symptoms were present, we looked at the histogram of Shannon index values for our 121 urine samples all together (see Figure 6).
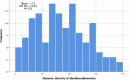
Figure 6
Shannon Index Values.
We did not carry out the planned mixed effects linear regression model to test the individual (main) and interaction effects of the response group (based on our 10% definition) and time on the Shannon index because only 11 individuals provided urine samples both before and after instillation (ie, there was an insufficient sample size for robust estimation in regression). Moreover, “response to LGG” is defined based on multiple samples (an across-samples summary), whereas the estimation of a Shannon index is based on individual samples (ie, Shannon index is a within-sample summary). Thus, response and Shannon index were not compatible summaries for a single analysis, and our sample was too small to explore alternative modeling methods. Instead of the planned inferential analyses, we descriptively summarized the diversity within individuals at each available time point. The frequency distribution in Figure 6 shows that the mean of observed Shannon index values was 1.7 (SD, 0.96). Shannon index values ≤1.5 suggest there may be rare organisms that bias the index values. Thus, although we had planned to use the Shannon index, we opted to summarize diversity with a different index that is nonparametric and corrects for rareness among microbes/organisms in the microbiome: The ACE estimates richness in any sample corrected by the number of observations (from that sample). We characterized the individual at the time of the urine sample as either symptomatic (USQNB-IC total, ≥1) or asymptomatic (USQNB-IC total, 0) and plotted the ACE values for each individual sample across observations, depending on whether they were symptomatic or not (Figure 7).
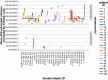
Figure 7
ACE Index Over Time.
Figure 7 suggests that the variation in ACE observed in the 21 urine samples contributed by 9 different individuals while they were symptomatic (top panel) was quite low—ranging from near 0 to 175. In contrast, the range of ACE values observed in urine samples from individuals when they were asymptomatic tended to have a wide range with a low near 0 and highs near 250 across individuals. Within-person variability was also high for individuals who contributed urine samples when they were asymptomatic; several samples from the same individual ranged from at least 50 to >200, while for those contributing multiple samples while symptomatic, the observed range was roughly 50 to 175. Figure 7 shows not only that there is not sufficient data for statistical inferences but also that our asymptomatic participants (bottom panel) exhibited a much greater range of variability both within and across persons than those who were symptomatic when their urine sample was collected.
We proposed to determine the magnitude of differences in microbiota between the response groups using the Yue-Clayton θ similarity index, which is a between-samples-based (ie, multiple samples) summary of species proportions. However, we were unable to obtain θ for our data because we had so few urine samples from both before and after instillation that met the “response to LGG” criteria. Instead, to explore between-sample diversity, we used a rough estimate of diversity, operational taxonomic unit (OTU), between samples obtained from symptomatic and asymptomatic individuals. In 121 samples, 21 were taken from individuals (multiple samples from 9 individuals) who were symptomatic, while 100 samples were from individuals in an asymptomatic state. The OTU counts, representing the number of distinct species found in any sample, did not differ significantly depending on whether the sample was obtained with or without symptoms (t[119] = 1.36, P = .18). Further, Lactobacillus was present, but there was no significant change pre- vs postinstillation. Technically, because the majority of samples were from the same individual, these t tests failed to meet the critical assumption of independent observations.
Overall, our initial analyses suggest no evidence of an association between either diversity estimates or OTU as summaries of the complexity of the urinary microbiome and symptomaticity. Because of the limitations in our data, these analyses cannot be interpreted clinically but instead suggest that more research will need to be done to glean clinical insights about symptomaticity from metagenomics results, particularly when those results yield a single-value summary.
Discussion
In this PCORI-sponsored study we did the following:
- Developed a patient-centered, patient-oriented outcome instrument, the USQNB-IC, using patient-centric methods also developed in this work
- Validated the USQNB-IC using a national sample of representative patients with NB who use IC
- Developed a self-management protocol for when to instill a probiotic (SMP-Pro) intravesically as triggered by selected urinary symptoms among the target population using a patient-centric approach
- Performed the first assessment of self-instilled intravesical LGG probiotic use among people with NB who use IC
- Showed that 1 to 2 doses of intravesical LGG probiotic instilled within 30 hours in response to cloudy and/or more foul-smelling urine, is safe and well-tolerated among people with NB who use IC
- Showed that study participants were satisfied with use of 1 to 2 doses of intravesical LGG probiotic instilled within 30 hours in response to cloudy and/or more foul-smelling urine
- Presented preliminary evidence that 1 to 2 doses of intravesical LGG probiotic instilled within 30 hours in response to cloudy and/or more foul-smelling urine may reduce urinary symptoms in adults with NB who use IC
These results are significant for multiple reasons.
Importance of the USQNB-IC
To date, there has not been an instrument to measure and monitor the universe of urinary symptoms indicative of UTIs among this population. Because of our inability to reliably measure and monitor urinary symptoms among people with NB, any potential study of a prophylactic or therapeutic agent is confounded due to a lack of ability to reliably measure symptoms.
As stated in Tractenberg et al,58 “this instrument is substantially (and substantively) different from any other: it was developed using explicitly patient-centric methods, is specific to the neurogenic bladder population, and the focus of these items is on infection-related symptoms.” Urinary symptom tools and health quality-of-life scales generally fall under the domains of female/general urinary incontinence, male urinary symptoms, and specific urinary symptoms (nocturia, overactive bladder, urgency, patient perception of bladder condition, and IC).53 A recent systematic review of patient-reported outcome measures for NB and bowel revealed heterogeneity in the current patient-reported outcome measures in this area and a clear focus on quality of life.68 Although the Medical Outcomes Study 36-Item Short Form survey was the most frequently used tool identified in this review, only 3 bladder- or bowel-specific measures were identified: the Qualiveen, Fecal Incontinence and Constipation Quality of Life, and the Quality of Life Scoring Tool Relating to Bowel Management, all of which are quality of life, and not symptom, measures.54 Another systematic review examined and compared validated questionnaires for people with NB due to SCI and MS. Of 18 questionnaires identified, 14 were for people with MS, 3 for people with SCI, and 1 was general.55
The only peer-reviewed, published symptom scale that we identified for people with NB is the NBSS.40,53 In the development of this generic scale, 266 items were generated from a search of the existing/published (not specific to NB) measurement tools. Of these, only 2 were specific to UTI. A multidisciplinary team reduced the original set of 266 items, and interviews were conducted with people with SCI and MS who identified urinary incontinence, UTI, urgency, and bladder spasms to be dominant issues for them.53 Although our instrument (the USQNB-IC) is also specific to individuals with NB, it differs significantly from the NBSS in terms of the origins of our items and their scope. As noted, the development of the USQNB-IC was fundamentally different from that of the NBSS, as our core information/symptoms are based on patient reports, as opposed to deriving from clinician- or evidence-defined reports.56
Self-management Protocol Using Probiotics
As stated in Groah et al,66 “we demonstrate that 1 or 2 doses of intravesical LGG self-instilled within a 22- to 30-hour period in response to urine that is more cloudy and/or foul-smelling, is safe and well-tolerated among adults and children with neurogenic lower urinary tract dysfunction (NLUTD) who manage their bladders with IC.” The study also provides preliminary demonstration that the SMP-Pro, which directs people with NLUTD when to instill or not to instill LGG, is usable by patients. Because we learned that assessment of symptoms must be done in real time with the SMP-Pro to determine more precisely whether there are difficulties in using it as a self-management tool, further studies are needed to generate estimates of correct/incorrect implementation of the protocol itself. Our conclusions are supported by the following observations in our study:
- AEs plus SAEs occurred throughout the study at a rate that did not associate with LGG use.
- AEs plus SAEs that occurred in instillers varied across and within people with NB.
- Rates of AEs plus SAEs were highest in P1 (baseline) and subsequently decreased.
- Generally, the rate of AEs plus SAEs did not increase with exposure to LGG.
- There were 1, 2, and 5 GU infectious (the most likely to be related to the intervention) AEs plus SAEs that occurred ≤1 week, 1 to 2 weeks, or >2 weeks, respectively, following instillation.
- There were only 3 each GU infectious AEs plus SAEs that occurred after 1 of the 357 total instillations (1.7%).
- Of 357 total instillations, only 1 (0.28%) resulted in irritation and withdrawal from the study.
- An independent DSMB determined that SAEs occurring after LGG instillation were “unlikely to be related to the intervention.”
This work is significant, because to date there has been no evidence demonstrating safety, tolerability, participant satisfaction, and effectiveness of a nonprescription, nonantibiotic self-management technique that those with NB can use to ameliorate bothersome urinary symptoms. For example, there have been poor-quality (and few) studies examining oral vitamin C, probiotics, cranberry products, and others, none of which were found to be effective (possibly or in part due to the gaps in measurement described previously).42-44,57 Evidence for prophylactic antibiotics, either oral or intravesical, does demonstrate reduced occurrence of UTIs, but is significantly associated with adverse effects due to the antibiotic and increases the risk of antibiotic resistance. Intravesical LGG might offer individuals with NB a means to self-manage their own frequent and burdensome urinary symptoms without excessive reliance on the health care system if future randomized trials can establish its efficacy.42-44,57
Future Research
As stated previously, we used “urinary symptoms” as a primary outcome, because using “UTI” would be confounded by diagnostic variability. We did demonstrate preliminary evidence of effectiveness (reduced urinary symptoms over time) and participant satisfaction in adults with NB who use IC. The safety and tolerability results support further research examining dosing (that work is currently in progress) and expansion to subpopulations with NB who use other forms of bladder management. As such, these results are not immediately generalizable to all people with NB or to those with NB who use differing bladder management methods. Recommendations for future research include assessment of varying doses of intravesical LGG over the same amount of time, greater amount of time, and perhaps periodically as a prophylactic agent. Different probiotics should be assessed for equivalence (or not), and different populations (those with NB who use indwelling catheterization) should be assessed. As a result of this study, we cannot draw any conclusions regarding self-instilled intravesical LGG in children other than that preliminary evidence from this trial does suggest that it is safe and well-tolerated when administered once or twice in a 30-hour period. For all individuals potentially using this approach, we would recommend continued use of our exclusion criteria, which includes but is not limited to immune deficiencies, known GU structural abnormalities, cancer, etc.
Subpopulation Considerations
This work was performed in a subpopulation of people with NB due to SCI, SB, and MS who manage their bladders with IC. The results are not generalizable to other populations with NB (for example, diabetes mellitus) and to people who use IC due to anatomic or structural problems, nor do we think those populations might be good targets for future research. When broadening this research to other populations, strong consideration should be given to risk of LGG infection due to the intervention within specific populations.
Study Limitations
First, because this was a first-in-human intervention trial, we strictly controlled the scope and breadth of the study intervention and study population. This was done to minimize potential risk. Second, we did not enroll the number of children planned and therefore can only draw very preliminary safety and tolerability conclusions about the intervention in that population. Low sample size of the child and MS populations limit conclusions in these populations specifically. The adult population was larger, and we are more confident in our conclusions in this population. Third, the population under study was limited by not including those with other structural changes to the GU tract, immune disorders, cancer, etc. Fourth, we could not measure the sensitivity and specificity of the urinary symptoms for detecting UTI. Fifth, we did not try to detect treatment response heterogeneity because of lack of statistical power due to the small number of participants with both pre- and postinstillation measure effect. Sixth, we had an insufficient number of individuals with both pre- and postinstillation urine samples (n = 11) to formally test hypotheses about effects revealed in urine. Similarly, data collection and urine sampling need to be done in real-time to allow for a better understanding of duration of beneficial effects and interpretation of relationships. Seventh, we could not form any conclusions about the effectiveness of the intravesical LGG instillations because our study design was quasi-experimental with no randomization to instillation or no instillation and consequently no control group. Eighth, because our study focused on patients who use IC, our results may not generalize to patients with indwelling catheters. The latter group warrants further research, as their symptom profiles and instillation protocols likely differ. Finally, we did not demonstrate LGG colonization after instillation and probiotic viability.
Conclusions
We developed the USQNB-IC specifically as a PC-PRO, and so it is consistent with principles of “valuing the patient perspective” and maintaining a “culture of patient centeredness in research.”58 However, this patient-centered approach is inconsistent with formal methods for instrument development (ie, those laid out by Bollen)69 that lead to the very measurement properties that are of greatest interest (as per Mokkink et al).60 Our work in this area (urinary symptom detection and management for people with NB) has balanced these 2 perspectives, and our preliminary validity evidence suggests that both can be integrated to yield a valid PC-PRO instrument.
We also demonstrated that 2 doses of intravesical LGG self-administered within a 22- to 30-hour period in response to cloudier or more bad-smelling, stronger, fouler or more-pungent-than-normal urine and in the absence of symptoms suggestive of infection, is safe and well-tolerated among people with NLUTD who manage their bladders with IC. We hypothesize that cloudy, more pungent urine represents a potentially preinfectious, transitional state of bladder inflammation that may respond to bacterial interference (the concept of low-virulence bacterial species interfering with colonization by other, more virulent, bacterial species). Despite preliminary evidence (instillers but not noninstillers had decreased symptom burden in the washout phase compared with the intervention phase) that intravesical Lactobacillus may reduce urinary symptom burden, we do not propose routine use of self-management of urinary symptoms or treatment of UTIs with probiotics at this time. Future research is underway to determine intravesical dosing in this patient population as well in an expanded one.
References
- 1.
- Stöhrer M, Blok B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56(1):81-88. doi:10.1016/j.eururo.2009.04.028 [PubMed: 19403235] [CrossRef]
- 2.
- Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. doi:10.1086/650482 [PubMed: 20175247] [CrossRef]
- 3.
- Forster CS, Hseieh M, Perez-Losada M, et al. A single intravesical instillation of probiotics is safe in children and adults with neuropathic bladder: a phase Ia clinical trial. J Spinal Cord Med. 2019;17(1-9). doi:10.1080/10790268.2019.1616456 [PMC free article: PMC7919893] [PubMed: 31100050] [CrossRef]
- 4.
- Litza JA, Brill JR. Urinary tract infections. Prim Care. 2010;37(3):491-507, viii. doi:10.1016/j.pop.2010.04.001 [PubMed: 20705195] [CrossRef]
- 5.
- Groah SL, Lammertse DP. Factors associated with survival after bladder cancer in spinal cord injury. J Spinal Cord Med. 2003;26(4):339-344. [PubMed: 14992334]
- 6.
- Filler G, Gharib M, Casier S, Lödige P, Ehrich JHH, Dave S. Prevention of chronic kidney disease in spina bifida. Int Urol Nephrol. 2012;44(3):817-827. doi:10.1007/s11255-010-9894-5 [PubMed: 21229390] [CrossRef]
- 7.
- Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85(11):1757-1763. [PubMed: 15520970]
- 8.
- Bølling Hansen R, Biering-Sørensen F, Kvist Kristensen J. Urinary calculi following traumatic spinal cord injury. Scand J Urol Nephrol. 2007;41(2):115-119. doi:10.1080/00365590600991383 [PubMed: 17454949] [CrossRef]
- 9.
- Chen Y, DeVivo MJ, Roseman JM. Current trend and risk factors for kidney stones in persons with spinal cord injury: a longitudinal study. Spinal Cord. 2000;38(6):346-353. [PubMed: 10889563]
- 10.
- Groah SL, Weitzenkamp DA, Lammertse DP, Whiteneck GG, Lezotte DC, Hamman RF. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83(3):346-351. [PubMed: 11887115]
- 11.
- Cardenas DD, Moore KN, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PM R. 2011;3(5):408-417. doi:10.1016/j.pmrj.2011.01.001 [PubMed: 21570027] [CrossRef]
- 12.
- Dicianno BE, Wilson R. Hospitalizations of adults with spina bifida and congenital spinal cord anomalies. Arch Phys Med Rehabil. 2010;91(4):529-535. doi:10.1016/j.apmr.2009.11.023 [PMC free article: PMC8474055] [PubMed: 20382283] [CrossRef]
- 13.
- Lijing O, Grosse SD, Thibadeau J, Swanson M, A Campbell VA. Outpatient medical conditions among children and adults with spina bifida in the United States: frequency and expenditures. J Pediatr Rehabil Med. 2010;3(3):177-185. doi:10.3233/PRM-2010-0127 [PubMed: 21791849] [CrossRef]
- 14.
- Consortium for Spinal Cord Medicine, Paralyzed Veterans of America. Bladder Management Following Spinal Cord Injury: What You Should Know. 2010. Accessed August 24, 2018. https://pva-cdnendpoint.azureedge.net/prod/libraries/media/pva/library/publications/consumer_guide_bladder_071410.pdf
- 15.
- DeJong G, Tian W, Hsieh C-H, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94(4):S87-S97. doi:10.1016/j.apmr.2012.10.037 [PubMed: 23527776] [CrossRef]
- 16.
- Haisma JA, van der Woude LH, Stam HJ, et al. Complications following spinal cord injury: occurrence and risk factors in a longitudinal study during and after inpatient rehabilitation. J Rehabil Med. 2007;39(5):393-398. doi:10.2340/16501977-0067 [PubMed: 17549331] [CrossRef]
- 17.
- Plowman R, Graves N, Esquivel J, Roberts JA. An economic model to assess the cost and benefits of the routine use of silver alloycoated urinary catheters to reduce the risk of urinary tract infections in catheterized patients. J Hosp Infect. 2001;48(1):33-42. doi:10.1053/jhin.2001.0938 [PubMed: 11358469] [CrossRef]
- 18.
- Hollenbeak CS, Schilling AL. The attributable cost of catheter-associated urinary tract infections in the United States: a systematic review. Am J Infect Control. 2018;46(7):751-757. doi:10.1016/j.ajic.2018.01.015 [PubMed: 29478760] [CrossRef]
- 19.
- Vaidyanathan S, Soni BM, Dundas S, Krishnan KR. Urethral cytology in spinal cord injury patients performing intermittent catheterisation. Paraplegia. 1994;32(7):493-500. doi:10.1038/sc.1994.78 [PubMed: 7970852] [CrossRef]
- 20.
- Schlager TA, Grady R, Mills SE, Hendley JO. Bladder epithelium is abnormal in patients with neurogenic bladder due to myelomeningocele. Spinal Cord. 2004;42(3):163-168. doi:10.1038/sj.sc.3101565 [PubMed: 15001981] [CrossRef]
- 21.
- Groah S, Perez-Losada M, Caldovic L, et al. MP20-08 pyuria and asymptomatic bacteriuria is associated with novel and specific urine microbiomes. J Urol. 2015;193(4):e226. doi:10.1016/j.juro.2015.02.980 [CrossRef]
- 22.
- Jayawardena V, Midha M. Significance of bacteriuria in neurogenic bladder. J Spinal Cord Med. 2004;27(2):102-105. [PubMed: 15162878]
- 23.
- The prevention and management of urinary tract infections among people with spinal cord injuries. National Institue on Disability and Rehabilitation Research Consensus Statement January 27-29, 1992. J Am Paraplegia Soc. 1992;15(3):194-204. doi:10.1080/01952307.1992.11735873 [PubMed: 1500945] [CrossRef]
- 24.
- Vickrey BG, Shekelle P, Morton S, Clark K, Pathak M, Kamberg C. Prevention and Management of Urinary Tract Infections in Paralyzed Persons: Summary. Agency for Healthcare Research and Quality; 1999. Accessed July 6, 2018. https://www
.ncbi.nlm .nih.gov/books/NBK11881/ [PMC free article: PMC4781422] [PubMed: 11487801] - 25.
- Tenke P, Kovacs B, Bjerklund Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008;31(Suppl 1):S68-S78. doi:10.1016/j.ijantimicag.2007.07.033 [PubMed: 18006279] [CrossRef]
- 26.
- US Department of Health and Human Services. Health care-associated infections. Updated January 15, 2020. Accessed July 6, 2018. https://health
.gov/hcq/prevent-hai.asp - 27.
- Madden-Fuentes RJ, McNamara ER, Lloyd JC, et al. Variation in definitions of urinary tract infections in spina bifida patients: a systematic review. Pediatrics. 2013;132(1):132-139. doi:10.1542/peds.2013-0557 [PubMed: 23796735] [CrossRef]
- 28.
- Clayson D, Wild D, Doll H, Keating K, Gondek K. Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): the UTI Symptom Assessment questionnaire. BJU Int. 2005;96(3):350-359. doi:10.1111/j.1464-410X.2005.05630.x [PubMed: 16042729] [CrossRef]
- 29.
- Miyazato M, Tana T, Higa A, et al. A questionnaire survey to assess lower urinary tract symptoms in patients with chronic stroke. Neurourol Urodyn. 2017;36(7):1890-1895. doi:10.1002/nau.23206 [PubMed: 28169449] [CrossRef]
- 30.
- Barry MJ, Fowler FJ, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564. [PubMed: 1279218]
- 31.
- Donovan JL, Abrams P, Peters TJ, et al. The ICS-‘BPH’ Study: the psychometric validity and reliability of the ICSmale questionnaire. Br J Urol. 1996;77(4):554-562. [PubMed: 8777617]
- 32.
- Donovan JL, Peters TJ, Abrams P, Brookes ST, de aa Rosette JJ, Schäfer W. Scoring the short form ICSmaleSF questionnaire. International Continence Society. J Urol. 2000;164(6):1948-1955. [PubMed: 11061889]
- 33.
- Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14(2):131-139. [PubMed: 7780440]
- 34.
- Litwin MS, McNaughton-Collins M, Fowler FJ, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999;162(2):369-375. [PubMed: 10411041]
- 35.
- O'Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(Suppl 5A):58-63. [PubMed: 9146003]
- 36.
- Cardozo L, Coyne KS, Versi E. Validation of the urgency perception scale. BJU Int. 2005;95(4):591-596. doi:10.1111/j.1464-410X.2005.05345.x [PubMed: 15705086] [CrossRef]
- 37.
- Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174(2):604-607. doi:10.1097/01.ju.0000165461.38088.7b [PubMed: 16006914] [CrossRef]
- 38.
- Homma Y, Yoshida M, Yamanishi T, Gotoh M. Core Lower Urinary Tract Symptom score (CLSS) questionnaire: a reliable tool in the overall assessment of lower urinary tract symptoms. Int J Urol. 2008;15(9):816-820. doi:10.1111/j.1442-2042.2008.02121.x [PubMed: 18657204] [CrossRef]
- 39.
- Abdelwahab O, Mohamed A, Mohamed T, Abdelzaher M. Outcomes of the Urogenital Distress Inventory (UDI-6) for 20- to 50-year-old females with lower urinary tract dysfunction in Qalubia Governorate, Egypt. UroToday Int J. 2011;04(1):art6. doi:10.3834/uij.1944-5784.2011.02.06 [CrossRef]
- 40.
- Reuvers SH, Korfage IJ, Scheepe JR, ’t Hoen LA, Sluis TA, Blok BF. The validation of the Dutch SF-Qualiveen, a questionnaire on urinary-specific quality of life, in spinal cord injury patients. BMC Urol. 2017;17(1):88. doi:10.1186/s12894-017-0280-9 [PMC free article: PMC5605985] [PubMed: 28927392] [CrossRef]
- 41.
- Welk B, Morrow S, Madarasz W, Baverstock R, Macnab J, Sequeira K. The validity and reliability of the neurogenic bladder symptom score. J Urol. 2014;192(2):452-457. doi:10.1016/j.juro.2014.01.027 [CrossRef]
- 42.
- Welk B, Morrow S, Madarasz W, Potter P, Sequeira K. The conceptualization and development of a patient-reported neurogenic bladder symptom score. Res Rep Urol. 2013;5:129-137. doi:10.2147/RRU.S51020 [PMC free article: PMC3826942] [PubMed: 24400244] [CrossRef]
- 43.
- Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10(1):174. doi:10.1186/1479-5876-10-174 [PMC free article: PMC3511201] [PubMed: 22929533] [CrossRef]
- 44.
- Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50(4):1376-1383. doi:10.1128/JCM.05852-11 [PMC free article: PMC3318548] [PubMed: 22278835] [CrossRef]
- 45.
- Toh S-L, Boswell-Ruys CL, Lee BSB, Simpson JM, Clezy KR. Probiotics for preventing urinary tract infection in people with neuropathic bladder. Cochrane Database Syst Rev. 2017;(9):CD010723. doi:10.1002/14651858.CD010723.pub2 [PMC free article: PMC6483743] [PubMed: 28884476] [CrossRef]
- 46.
- Darouiche RO, Thornby JI, Cerra-Stewart C, Donovan WH, Hull RA. Bacterial interference for prevention of urinary tract infection: a prospective, randomized, placebo-controlled, double-blind pilot trial. Clin Infect Dis. 2005;41(10):1531-1534. doi:10.1086/497272 [PubMed: 16231269] [CrossRef]
- 47.
- Darouiche RO, Green BG, Donovan WH, et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology. 2011;78(2):341-346. doi:10.1016/j.urology.2011.03.062 [PubMed: 21683991] [CrossRef]
- 48.
- Sundén F, Håkansson L, Ljunggren E, Wullt B. Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J Urol. 2010;184(1):179-185. doi:10.1016/j.juro.2010.03.024 [PubMed: 20483149] [CrossRef]
- 49.
- Cadieux PA, Burton J, Devillard E, Reid G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J Physiol Pharmacol. 2009;60(Suppl 6):13-18. [PubMed: 20224146]
- 50.
- Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11(9):821-834. doi:10.1080/17474124.2017.1343143 [PMC free article: PMC6104804] [PubMed: 28650209] [CrossRef]
- 51.
- Gao J, Li Y, Wan Y, et al. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol. 2019;10:477. doi:10.3389/fmicb.2019.00477 [PMC free article: PMC6426789] [PubMed: 30923519] [CrossRef]
- 52.
- de Llano DG, Arroyo A, Cárdenas N, Rodríguez JM, Moreno-Arribas MV, Bartolomé B. Strain-specific inhibition of the adherence of uropathogenic bacteria to bladder cells by probiotic Lactobacillus spp. Pathog Dis. 2017;75(4). doi:10.1093/femspd/ftx043 [PubMed: 28402532] [CrossRef]
- 53.
- Cirl C, Wieser A, Yadav M, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008;14(4):399-406. doi:10.1038/nm1734 [PubMed: 18327267] [CrossRef]
- 54.
- Karlsson M, Scherbak N, Khalaf H, Olsson P-E, Jass J. Substances released from probiotic Lactobacillus rhamnosus GR-1 potentiate NF-κB activity in Escherichia coli-stimulated urinary bladder cells. FEMS Immunol Med Microbiol. 2012;66(2):147-156. doi:10.1111/j.1574-695X.2012.00994.x [PubMed: 22620976] [CrossRef]
- 55.
- McMillan A, Dell M, Zellar MP, et al. Disruption of urogenital biofilms by lactobacilli. Colloids Surf B Biointerfaces. 2011;86(1):58-64. doi:10.1016/j.colsurfb.2011.03.016 [PubMed: 21497071] [CrossRef]
- 56.
- Shim YH, Lee SJ, Lee JW. Antimicrobial activity of Lactobacillus strains against uropathogens. Pediatr Int. 2016;58(10):1009-1013. doi:10.1111/ped.12949 [PubMed: 26865336] [CrossRef]
- 57.
- Seow SW, Rahmat JNB, Mohamed AAK, Mahendran R, Lee YK, Bay BH. Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium bovis (bacillus Calmette-Guerin). J Urol. 2002;168(5):2236-2239. doi:10.1097/01.ju.0000034353.97729.69 [PubMed: 12394766] [CrossRef]
- 58.
- Tractenberg RE, Groah SL, Rounds AK, Ljungberg IH, Schladen MM. Preliminary validation of a urinary symptom questionnaire for individuals with neuropathic bladder using intermittent catheterization (USQNB-IC): a patient-centered patient reported outcome. PLoS One. 2018;13(7):e0197568. doi:10.1371/journal.pone.0197568 [PMC free article: PMC6038997] [PubMed: 29990375] [CrossRef]
- 59.
- QSR International. Fueling medical research. Accessed June 27, 2016. https://www
.qsrinternational .com/nvivo-qualitative-data-analysis-software /about/nvivo /who-its-for/health - 60.
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737-745. doi:10.1016/j.jclinepi.2010.02.006 [PubMed: 20494804] [CrossRef]
- 61.
- CITI Program. Collaborative Institutional Training Initiative. Accessed December 22, 2020. https://www
.citiprogram.org/ - 62.
- van Nieuwkoop C, den Exter PL, Elzevier HW, den Hartigh J, van Dissel JT. Intravesical gentamicin for recurrent urinary tract infection in patients with intermittent bladder catheterisation. Int J Antimicrob Agents. 2010;36(6):485-490. doi:10.1016/j.ijantimicag.2010.05.005 [PubMed: 20580533] [CrossRef]
- 63.
- Grabnar I, Bogataj M, Belic A, Logar V, Karba R, Mrhar A. Kinetic model of drug distribution in the urinary bladder wall following intravesical instillation. Int J Pharm. 2006;322(1-2):52-59. doi:10.1016/j.ijpharm.2006.05.026 [PubMed: 16806751] [CrossRef]
- 64.
- US Food and Drug Administration Home Page. Accessed December 13, 2018. https://www
.fda.gov/ - 65.
- Tractenberg RE, Groah SL, Rounds AK, et al. Clinical profiles and symptom burden estimates to support decision-making using the Urinary Symptom Questionnaire for people with Neurogenic Bladder (USQNB) using intermittent catheters. PM R. 2020. Online ahead of print. doi:10.1002/pmrj.12479 [PubMed: 32860333] [CrossRef]
- 66.
- Groah SL, Rounds AK, Ljungberg IH, Sprague BM, Frost JK, Tractenberg RE. Intravesical Lactobacillus rhamnosus GG is safe and well tolerated in adults and children with neurogenic lower urinary tract dysfunction: first-in-human trial. Ther Adv Urol. 2019;11:1756287219875594. doi:10.1177/1756287219875594 [PMC free article: PMC6777056] [PubMed: 31620195] [CrossRef]
- 67.
- US Food and Drug Administration. CFR - Code Of Federal Regulations Title 21. Updated April 1, 2020. Accessed June 5, 2019. https://www
.accessdata .fda.gov/scripts/cdrh /cfdocs/cfcfr/cfrsearch.cfm?fr=312.32 - 68.
- Patel DP, Elliott SP, Stoffel JT, Brant WO, Hotaling JM, Myers JB. Patient reported outcomes measures in neurogenic bladder and bowel: a systematic review of the current literature. Neurourol Urodyn. 2016;35:8-14. [PubMed: 25327455]
- 69.
- Bollen KA. Structural Equations With Latent Variables. Wiley; 1989.
Related Publications
- Tractenberg RE, Garver A, Ljungberg IH, Schladen MM, Groah SL. Maintaining primacy of the patient perspective in the development of patient-centered patient reported outcomes. PLoS One. 2017;12(3):e0171114. doi:10.1371/journal.pone.0171114 [PMC free article: PMC5336216] [PubMed: 28257414] [CrossRef]
- Tractenberg RE, Groah SL, Rounds AK, Ljungberg IH, Schladen MM. Preliminary validation of urinary symptoms questionnaire for individuals with neuropathic bladder using intermittent catheterization (USQNB-IC). A patient-centered patient reported outcome. PLoS One. 2018;13(7):e0197568. doi:10.1371/journal.pone.0197568 [PMC free article: PMC6038997] [PubMed: 29990375] [CrossRef]
- Forster C, Hsieh M, Pérez-Losada M, et al. A single intravesical instillation of Lactobacillus rhamnosus GG is safe in children and adults with neuropathic bladder: a phase 1a clinical trial. J Spinal Cord Med. 2019;1-8. doi:10.1080/10790268.2019.1616456. [PMC free article: PMC7919893] [PubMed: 31100050] [CrossRef]
- Groah SL, Rounds AK, Ljungberg IH, Sprague BM, Frost JK, Tractenberg RE. Intravesical Lactobacillus rhamnosus GG is safe and well tolerated in adults and children with neurogenic lower urinary tract dysfunction: first-in-human trial. Ther Adv Urol. 2019;11:1756287219875594. doi:10.1177/1756287219875594 [PMC free article: PMC6777056] [PubMed: 31620195] [CrossRef]
- Schladen M, McManus T, Claypool H, Bennewith A, Groah S. Intermittent catheter reimbursement in the United States: the experience of nine stakeholders through the lens of Actor-Network Theory. Submitted to Disability Studies Quarterly on September 30, 2018.
- Pohl HG, Groah SL, Pérez-Losada M, et al. The urine microbiome of healthy men and women differs by urine collection method. Int Neurol J. 2020;24(1):41-51. doi:10.5213/inj.1938244.122 [PMC free article: PMC7136448] [PubMed: 32252185] [CrossRef]
- Tractenberg RE, Groah SL, Rounds AK, et al. Clinical profiles and symptom burden estimates to support decision-making using the Urinary Symptom Questionnaire for people with Neurogenic Bladder (USQNB) using intermittent catheters. PM R. 2020. Online ahead of print. doi:10.1002/pmrj.12479 [PubMed: 32860333] [CrossRef]
Acknowledgments
We would like to thank the consumer experts who worked with the research team on this study: Elizabeth Davis, Henry Claypool, Harsh Thakkar, Terrence McManus, Jill Hill, and Cheryl Czekaj. This has been a rewarding learning experience for everyone involved, and the contributions of these experts were essential for this study's success
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#AD-1310-08215). Further information available at: https://www.pcori.org/research-results/2014/self-management-urinary-symptoms-using-probiotic-people-spinal-cord-injuries
Appendices
Appendix A.
Abbreviations (PDF, 136K)
Appendix B.
Urinary Symptoms Questionnaire for Neurogenic Bladder—Intermittent Catheterization (PDF, 398K)
Appendix C.
Preliminary Validation of the USQNB-IC: a PC-PRO (PDF, 1.8M)
Table 1. COSMIN20 criteria and how they were assessed in this study (PDF, 80K)
Table 2. COSMIN20 reliability evidence: Patient groups (PDF, 212K)
Fig 2. Bayesian network of USQNB-IC items, full sample (PDF, 1.2M)
Table 3. Item endorsement (in percent) by group (PDF, 80K)
Table 5. Proportions of respondents endorsing a USQ-NB item and not attributing it to a UTI (PDF, 255K)
Appendix D.
PCORI Safety Study Results (PDF, 152K)
Suggested citation:
Groah S, Ljungberg I, Tractenberg R, Rounds A. (2020). Self-Management of Urinary Symptoms Using a Probiotic in People with Spinal Cord Injuries, Spina Bifida, and Multiple Sclerosis. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/12.2020.AD.131008215
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Effects of Intravesical Lactobacillus Rhamnosus GG on Urinary Symptom Burden in People with Neurogenic Lower Urinary Tract Dysfunction.[PM R. 2021]Effects of Intravesical Lactobacillus Rhamnosus GG on Urinary Symptom Burden in People with Neurogenic Lower Urinary Tract Dysfunction.Tractenberg RE, Groah SL, Frost JK, Rounds AK, Davis E, Ljungberg IH, Schladen MM. PM R. 2021 Jul; 13(7):695-706. Epub 2020 Nov 10.
- Intravesical Lactobacillus rhamnosus GG is safe and well tolerated in adults and children with neurogenic lower urinary tract dysfunction: first-in-human trial.[Ther Adv Urol. 2019]Intravesical Lactobacillus rhamnosus GG is safe and well tolerated in adults and children with neurogenic lower urinary tract dysfunction: first-in-human trial.Groah SL, Rounds AK, Ljungberg IH, Sprague BM, Frost JK, Tractenberg RE. Ther Adv Urol. 2019 Jan-Dec; 11:1756287219875594. Epub 2019 Oct 3.
- Intravesical Lactobacillus rhamnosus GG Alters Urobiome Composition and Diversity Among People With Neurogenic Lower Urinary Tract Dysfunction.[Top Spinal Cord Inj Rehabil. 2...]Intravesical Lactobacillus rhamnosus GG Alters Urobiome Composition and Diversity Among People With Neurogenic Lower Urinary Tract Dysfunction.Groah SL, Rounds AK, Pérez-Losada M. Top Spinal Cord Inj Rehabil. 2023 Summer; 29(3):44-57. Epub 2023 Aug 16.
- Review Intravesical treatments for painful bladder syndrome/ interstitial cystitis.[Cochrane Database Syst Rev. 2007]Review Intravesical treatments for painful bladder syndrome/ interstitial cystitis.Dawson TE, Jamison J. Cochrane Database Syst Rev. 2007 Oct 17; (4):CD006113. Epub 2007 Oct 17.
- Review Systematic review and practice policy statements on urinary tract infection prevention in adults with spina bifida.[Transl Androl Urol. 2018]Review Systematic review and practice policy statements on urinary tract infection prevention in adults with spina bifida.Tradewell M, Pariser JJ, Nimeh T, Elliott SP, Neurogenic Bladder Research Group. Transl Androl Urol. 2018 May; 7(Suppl 2):S205-S219.
- Self-Management of Urinary Symptoms Using a Probiotic in People with Spinal Cord...Self-Management of Urinary Symptoms Using a Probiotic in People with Spinal Cord Injuries, Spina Bifida, and Multiple Sclerosis
Your browsing activity is empty.
Activity recording is turned off.
See more...
