This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
The overwhelming majority of people with Down syndrome (DS) do not have access to DS specialty clinics, a disparity resulting in delayed or missed diagnoses and significant untreated comorbidities. To meet this critical gap in needs, our team created Down Syndrome Clinic to You (DSC2U), a novel, web-based technology created for caregivers of individuals with DS, which collects an extensive array of symptoms and historical data and automatically generates personalized recommendations for the caregiver (Caregiver Checklist) and the patient's primary care provider (Primary Care Provider Plan).
Objectives:
We aimed (1) to test whether a customizable Caregiver Checklist and Primary Care Provider Plan vs usual care would increase caregiver-reported, provider-driven health actions consistent with national guidelines and (2) to determine whether a customizable Caregiver Checklist and Primary Care Provider Plan vs usual care would be satisfactory to caregivers and providers and could improve the quality of life (QOL) for caregivers/families.
Methods:
We conducted a national randomized controlled trial of 230 caregivers who had sons or daughters with DS. We sought to determine whether, compared with usual care, our personalized Caregiver Checklist and Primary Care Provider Plan would improve adherence to 5 health care evaluations consistent with national guidelines: celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation. Completions of these evaluations were assessed by parent report 7 months after a wellness visit with the primary care provider. To validate caregiver responses, we obtained medical records from 20% of the caregivers. To determine whether using DSC2U was satisfactory to caregivers and providers, we asked for their opinions on novel surveys. To assess whether DSC2U could improve the QOL for caregivers/families, we used nationally validated Pediatric Quality of Life (PedsQL) survey instruments.
Results:
Random assignment to the DSC2U resulted in significantly greater compliance with the 5 health care evaluations we assessed during a 7-month follow-up period. The number of indicated evaluations that were completed or that the primary care provider recommended was 1.6-fold greater among families offered use of the DSC2U than among those receiving standard of care (for the intervention group: mean [SD] number of evaluations that were recommended or completed, 0.53 [0.73] evaluations; indicated evaluations that were recommended or completed: 0 evaluations, 59.0%; 1 evaluation, 30.8%; 2 evaluations, 8.5%; and 3 evaluations, 1.7%; for the control group: mean [SD] number of evaluations that were recommended or completed, 0.33 [0.53] sessions; indicated evaluations that were recommended or completed: 0 evaluations, 69.9%; 1 evaluation, 27.4%; 2 evaluations, 2.7%; and 3 evaluations, 0%; difference = 0.20; 95% CI, 0.04-0.37; P = .016). Caregivers were highly accurate historians in reporting on the measured outcomes. Both caregivers and primary care providers reported high levels of satisfaction, even though caregiver QOL measures were not improved during this study.
Conclusions:
DSC2U improved adherence to the national DS health care guidelines in a modality that both caregivers and primary care providers valued highly. DSC2U was effective in bringing high-cost specialty-level care to low-cost primary care settings.
Limitations:
The participating caregivers were largely well educated, well resourced, and eager to access health information. To this extent, our results might not be generalizable to all families who have sons or daughters with DS.
Background
Families of people with Down syndrome often say they worry that the medical establishment has passed them by. They feel marginalized, unseen, and unserved. Families of people with Down syndrome don't want admiration, and they don't want pity. They feel a critical and urgent need for adequate health care for their loved ones.
— Patricia E. Bauer, caregiver
Down Syndrome Epidemiology and Health Services
Current estimates suggest that the prevalence of individuals with Down syndrome (DS) in the United States is approximately 212 000 and growing.1 This growth is owed mainly to the longer life span of these individuals, now approximately 60 years, and it is seen even with an increase in DS-related elective pregnancy terminations.1,2 Approximately 57% of people with DS are aged >20 years, and an estimated 28% are aged >40 years. Patients with DS are prone to multiple chronic conditions over their lifetime. Many of these can be prevented and even treated, including congenital heart disease, thyroid conditions, vision and hearing loss, gastrointestinal disorders, and obstructive sleep apnea.3
The American Academy of Pediatrics (AAP) published DS health supervision guidelines for primary care providers (PCPs), which include age-specific screening tests and procedures.3 DS experts also developed consensus-based guidelines to aid PCPs with the surveillance and management of the unique challenges faced by adults with DS.4-6 Studies, however, have demonstrated that the care patients with DS have received when managed by their PCPs alone adheres to only 10% to 67% of these national guidelines.7-10 Some technology-based interventions targeting physicians have been successful, but the gap between the expected care supported by national guidelines and the actual care delivered by PCPs continues to exist, suggesting that other stakeholders should also be targeted.10
Staying current on the growing complexities for this population can be both impractical and unreasonable for PCPs. To address this barrier to care, 71 DS specialty clinics have been formed in 34 US states11,12 since 1967. These clinics have proven to be helpful with the diagnosis and management of co-occurring conditions such as gastrointestinal disorders, behavioral diagnoses (eg, autism spectrum disorder), and neurologic conditions.8 Despite their documented impact, the 71 DS clinics combined can serve only <5% of the estimated population of people with DS in this country. Expanding this network is unlikely because these multidisciplinary outpatient clinics are becoming increasingly financially unsustainable within today's health care insurance models. Put simply, the overwhelming majority of people with DS do not have access to such DS specialty clinics, a disparity that can lead to delayed or missed diagnoses and significant untreated coexisting conditions.
Previous research from the general population suggests that directly educating and involving patients and/or family caregivers, especially when reinforced by discussion with clinicians, will improve quality, efficiency, and health outcomes.13 Specifically, technology-based solutions that combine health information with social support, decision support, or behavioral change support have been shown to improve knowledge, social support, health behaviors, uptake of screening, and a sense of self-efficacy in users.14 Most health technology applications are intended for use by patients, but preliminary studies of technology applications targeted to family or other informal caregivers of individuals with diverse conditions, including patients with intellectual disabilities,15 present a clear opportunity to address caregiver needs through technological innovations.
DSC2U: A Tool for Caregivers and PCPs
To meet this critical gap in needs, our team created Down Syndrome Clinic to You (DSC2U), a novel, web-based technology created for family or other informal caregivers of individuals with DS (see Appendix A). It collects an extensive array of symptoms and historical data and automatically generates personalized recommendations for the caregiver (Caregiver Checklist) and the patient's PCP Plan. To test whether this customized Caregiver Checklist and PCP Plan would increase health actions consistent with national guidelines, we conducted a national randomized controlled trial (RCT) using DSC2U as the intervention.
Aims of the Trial
The specific aims of our trial were as follows:
- Specific Aim 1 (primary outcome): To test whether a customizable Caregiver Checklist and PCP Plan vs usual care would increase caregiver-reported, provider-driven health actions consistent with national guidelines.
- Specific Aim 2 (secondary outcomes): To determine whether a customizable Caregiver Checklist and PCP Plan vs usual care would be satisfactory to caregivers and PCPs and could improve QOL for caregivers and families.
Patient and Stakeholder Engagement
Identifying a Need for DSC2U With Family Caregivers
The genesis of this study came from our team's collective listening to patients, caregivers, and other key stakeholders around the country about the needs of people with DS and their caregivers. During the 4 years preceding the receipt of this grant, Dr Brian Skotko, the principal investigator (PI), had given many presentations to patients with DS and their family members in national and international arenas. He was often invited to talk about the health care guidelines for patients with DS. Very frequently, he would hear from families, “I wish I had a clinic like yours nearby!”
Dr Skotko also had a following on his Facebook and Twitter pages. Daily, he would receive questions from parents and caregivers around the globe, asking him medical questions that were already answered in the national DS health care guidelines. Parents and caregivers lamented that they did not live in close proximity to a DS specialty clinic, and they would often ask how they could best prepare for their son's or daughter's PCP visit. Dr Skotko also has a sister with DS with no access to a DS specialty clinic. Therefore, he understood the importance of this need on both a professional and personal level.
These frequent comments were followed by more regular conversations with a select group of mothers of individuals with DS and patient advocates from the Massachusetts Down Syndrome Congress during the preparation of the original grant proposal. The research team identified these caregivers and patient advocates from the personal connections Dr Skotko had made over the years. Together, we shaped the framework of this proposal, planned the intervention, defined shared outcomes, and selected mutually relevant outcome measures. All caregivers and patient advocates who participated in these early meetings agreed to participate in a parent/caregiver working group (WG), working hand in hand with our research team on this project (Table 1).
Table 1
Family Caregiver WG.
Establishing and Working With Stakeholder WGs
During the early, pre-proposal discussions described previously, our parent/caregiver advisors shared that, for DSC2U to be most effective, engaging PCPs would be important. As a result, we also reached out to community-based PCPs to ensure that their needs would be represented in the resulting intervention. We wanted our PCP representatives to come from diverse locales and to practice in states not served by a specialty clinic. Four PCPs were identified through personal connections of Dr Alison Schwartz, a former PCP at Massachusetts General Hospital and one of the early investigators on this project. All PCPs made time available to review and comment on the proposal throughout the grant-writing period, and all agreed to be part of our PCP WG (Table 2).
Table 2
PCP WG.
Our third stakeholder group consisted of our content experts. These individuals—national leaders in DS care, DS patient advocacy, and shared health decision-making—made up our expert advisory panel (Table 3) and were personally invited by Dr Skotko.
Table 3
Expert Advisory Panel.
Throughout the 3-year research grant period, we met monthly via teleconference separately with each of our 3 WGs: (1) parent/caregivers, (2) PCPs, and (3) expert advisors. Our stakeholders were engaged in every aspect of the project. These included the following:
- Reviewing the wording, appropriateness, and order of the DSC2U online questionnaire
- Designing and creating content for DSC2U's Caregiver Checklist and PCP Plan
- Designing and authoring copy for the DSC2U research website, where potential participants would be directed to learn more about DSC2U and our study
- Selecting appropriate survey questions and validated surveys to assess outcome measures
Caregivers spent additional time outside the monthly meetings reviewing all study participant-facing copy and materials (eg, email reminders, survey materials, social media announcements). They also worked with us to develop our recruitment strategy and actively participated in the final phase of the recruitment period by making telephone calls on behalf of the study to local DS patient community groups to increase our representation of Black and Hispanic families. The expert advisory panel additionally worked with the research team to review the algorithms, or rule sets, that would drive the specific recommendations generated through DSC2U to create the personalized Caregiver Checklists and PCP Plans.
All WG members had opportunities to comment on and review the interim and final data analyses. Toward the end of our study, they were also particularly instructive in strategies to disseminate the findings within the DS community and to implement DSC2U as a self-sustaining, publicly available resource on completion of this grant.
Now that the study is finished, the parent/caregiver WG has remained committed to helping disseminate the results to the DS community. They have all volunteered to continue to meet by teleconference every other month to advise on how to disseminate and implement our results.
Evaluating Stakeholder Engagement
At the end of the project, we surveyed all stakeholders to gather feedback on their engagement in the project and to inform further efforts. The Patient Engagement in Research Scale (PEIRS) is a framework designed by Hamilton and colleagues at the University of British Columbia.16 We used this instrument to quantifiably assess meaningful patient engagement throughout the research process via a 37-item questionnaire measuring 8 organizing themes for meaningful patient partner engagement: procedural requirements, convenience, contributions, team environment, support, research environment, feeling valued, and benefits.
We used the final PEIRS questionnaire in its entirety. We made only slight wording changes to adapt the original questions, which addressed patients, to questions that addressed all our stakeholder WG members. Our research team, including survey scientists and nonparticipating primary care and expert colleagues, reviewed the PEIRS and felt that its face validity was appropriate for a task of this kind. Although Hamilton et al originally administered the PEIRS on paper at a stakeholder meeting, we administered the survey electronically in June and July 2019. All 23 members of our 3 WGs and our core research team members received an initial invitation and up to 3 reminders. Responses were anonymous.
We received 22 (95.7%) responses from the members of the research team invited to complete our PEIRS. The average total score on the PEIRS was 93.5 out of a maximum of 100. Although the total scores varied slightly among the 4 groups, they were all suggestive of strong engagement levels (Table 4). Experts ranked their experience in the DSC2U research process the most favorably (PEIRS total score, 97.7); PCPs had the lowest score, albeit still very favorable (PEIRS total score, 86.7).
Table 4
PEIRS Total Scores.
Respondents identified project strengths in verbatim response questions, which included the effective leadership of the PI: “[He] brought a great team of researchers and parents together to create a tool that is useful and beneficial to the community”; “The objective behind it was such a good one, it was hard not to be excited to be a part of it. The meetings were well organized and efficient and [the PI] is so incredibly positive that it is contagious.” Respondents also commented about the organization of the project: “The project was a very collaborative process. Attention to detail also a strength”; “Clear, concise communication. Strong respectful team with dedication to the project and purpose.”
We also asked respondents to identify areas for improvement. A few stakeholders in the external WGs suggested that holding meetings clustered around the needs of the project, rather than at predetermined intervals, might have been productive. Given the amount of work in the initial phases, some stakeholders suggested that having more frequent meetings early in the project would have been beneficial. Others commented that we could convene meetings less frequently once the study was under way, especially during the RCT period, when the project was running smoothly.
Methods
Study Overview
Our first aim and primary outcome was to determine, through a 2-arm, national RCT, whether the use of DSC2U would result in improved adherence to 5 health care evaluations (celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation), consistent with national guidelines. Individuals randomly assigned to the intervention group were compared with individuals who received usual care (the control group). In the second aim, our secondary outcome, we wanted to determine whether this Caregiver Checklist and PCP Plan resulted in greater caregiver and provider satisfaction and greater QOL than with usual care. The research plan, as detailed below, was approved by the Partners Human Research Committee.
Study Setting
Our study was national in scope and was specifically targeted toward caregivers of individuals with DS who did not have access to specialty DS care. We wanted to ensure that our study would evaluate the efficacy of the Caregiver Checklist and PCP Plan in the real-life settings in which they would ultimately be used. Throughout the study, we define “caregivers” as parents, siblings, or other persons responsible for the care of an individual with DS whom they identify as a “dependent.”
Participants
We enrolled caregivers from around the country between October 3, 2017, and September 30, 2018. Caregivers in the United States who had a child or other dependent with DS 1 year of age or older and who did not have access to a DS specialty clinic were eligible for this study. Because the primary outcome applied to all persons with DS beginning at the age of 1 year, eligibility was limited to those caregivers whose child or dependent with DS was 1 year of age or older. We specifically recruited caregivers and not patients because most people with DS have a mild to severe intellectual disability that can make providing detailed medical information challenging. In all cases, however, caregivers were encouraged to involve their child or dependent jointly in the process. We recruited both English-speaking and Spanish-speaking participants.
DS occurs naturally and proportionally in all races and ethnicities, so our population estimates were proportional to the racial/ethnic distribution of the US population as reported in the 2010 US Census. To achieve commensurate representation in our study, we applied a quota system in offering enrollment using the race and ethnicity of the individual with DS (not the caregiver). Based on an idealized enrollment table (Table 5), our plan was to enroll participants such that we had no more than 144 White individuals with DS, no fewer than 25 Hispanic or Latino/Latina individuals with DS, and no fewer than 20 Black individuals with DS. We also planned to enroll no more than 120 individuals with DS of 1 sex. These quotas proved to be important. We could have completed study enrollment in a few weeks without these quotas, but virtually all individuals with DS would have been White and non-Hispanic. National data still show a digital divide by race and ethnicity in terms of access to the internet and health information; setting enrollment targets allowed time to try to overcome these barriers.
Table 5
Idealized Enrollment Table.
We implemented several safety measures for patient data privacy. These included storing deidentified participant data in a password-protected database and limiting access to study data to only the core DSC2U study team. We will retain the data for at least 3 years after completion of the research, in addition to depositing the data in PCORI's data registry for future access.
We also wanted to ensure that participants had the opportunity to access all study materials in Spanish, if preferred. We translated study materials into Spanish after our Partners Human Research Committee approved the English version. Thus, the Spanish materials were available on a somewhat delayed schedule. Through careful discussion with our parent/caregiver WG and expert advisory panel, we developed a multitiered recruitment strategy through multiple mechanisms and media. These included the following:
- DS-Connect, a national contact registry available through the NIH, where parents/caregivers from around the country have indicated that they are interested in being contacted for potential research opportunities about DS. Melissa Parisi, MD, PhD, chief of the Intellectual and Developmental Disabilities Branch at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, was also a member of our expert advisory panel.
- e-newsletters, Facebook, and Twitter postings of DS advocacy groups around the country. Maureen Gallagher, MS, executive director of one of these groups, the Massachusetts Down Syndrome Congress, was on our expert advisory panel and helped us with outreach to her counterparts around the country.
- Our own Facebook and Twitter postings through the MGH Down Syndrome Program, which already had a well-established social media presence.
These resources directed potentially eligible participants to our study website (www.dsc2u.org) for the eligibility screening questionnaire in English and Spanish and online consent. After selecting a language preference (English or Spanish), in addition to questions about the child's or dependent's biological sex, race, and ethnicity, the eligibility screening questions included the following:
- Do you have a child or dependent with DS?
- Is your child or dependent aged 1 year or older?
- When is your child's next annual well visit (“primary care provider visit”)?
- Does your child or dependent currently receive care at a DS specialty clinic? (If the child or dependent was actively followed in a DS specialty clinic, even one out of state; for example, a family from Arizona who travels to Texas each year for their child to be seen in a DS specialty clinic would be ineligible.)
To be eligible, the caregiver needed to respond “yes” to questions 1 and 2 and “no” to question 4, and the child or dependent needed to fall within our enrollment quotas. Because of the study timeline (described below), the PCP visit needed to be scheduled no later than 11 months before the end of the grant period. The caregiver must also have provided a valid email address. We allowed only 1 patient's caregiver for each participating PCP because multiple patients seen by the same PCP would not have been independent events. In these cases, we offered eligibility on a first-come-first-serve basis. If the caregiver was considered eligible, he or she was then automatically taken to a web page to view our consent form.
Intervention and Control Groups
We defined our intervention and control groups as follows:
- Intervention group: Parents/caregivers who used the technological intervention as described below. That is, they completed DSC2U and subsequently received the customized Caregiver Checklist and PCP Plan.
- Control group: Parents/caregivers who received usual care (ie, the typical advice and recommendations the PCPs would offer). Participants randomly assigned to this group received access to the DSC2U intervention upon conclusion of the study, along with their personalized Caregiver Checklist and PCP Plan.
The intervention, DSC2U, is a web-based application for families to obtain up-to-date, personalized health and wellness information for their loved one with DS. When caregivers access DSC2U online, they are presented with an intake questionnaire in which they are asked to identify current symptoms in their loved one with DS along with any past medical or behavioral diagnoses and any recent blood work or diagnostic testing. DSC2U also contains optional questions about nutrition, education, therapies, life skills, and community resources.
At the core of DSC2U lies its algorithms, a set of rules that generates recommendations based on specific responses in the intake questionnaire. The rules are based on national guideline recommendations for DS care and expert consensus developed during the initial phase of this grant by the research team, refined with the assistance of the expert advisory panel. Upon submission of the intake questionnaire, the caregivers' responses are run through the DSC2U algorithm results in the Caregiver Checklist and PCP Plan, which are made available within seconds. The participant receives a notification email with a link to access their personalized Caregiver Checklist and PCP Plan.
Description of Caregiver Checklist
Our parent/caregiver WG helped develop and optimize the content for the Caregiver Checklist. This WG worked with the expert advisory panel and our research team to develop a clinically sound, parent-meaningful document. The Caregiver Checklist was a personalized 1- to 2-page summary report with health care recommendations for their child or dependent (Figure 1; Appendix Bi). Our parent/caregiver WG ensured that the Caregiver Checklist was empowering, direct, succinct, and, above all, affirming. These recommendations were auto-programmed based on the types of answers that respondents provided when completing DSC2U.

Figure 1
Caregiver Checklist Snapshot.
For example, if a caregiver of a teenager checked boxes that the individual was gasping, choking, and snorting at night and that the teen has never had a sleep study, these responses triggered a recommendation to discuss a sleep study with the PCP. In addition, not only did the caregivers receive the suggestion of a sleep study, they also received practical information on how to prepare for a sleep study when a participant has an intellectual disability. This information could be accessed by clicking on the green “Why?” pop-up question at the end of each recommendation. For example, if a caregiver is interested in understanding why the sleep study is recommended for their child or dependent, the green “Why?” will redirect them to a customized page of up-to-date information on sleep studies and people with DS. Similarly, if a respondent checked that a child or dependent with DS was experiencing frequent constipation, bloating, and behavioral problems, they received a recommendation to talk about celiac disease testing with the child's PCP, because this condition occurs at an increased frequency in patients with DS. The Caregiver Checklist also included tailored recommendations on books and other educational references and community resources that our WGs deemed to be relevant, accurate, and helpful.
All recruitment language was reviewed by a legal team at Massachusetts General Hospital (MGH) to ensure that participants were informed that DSC2U does not offer either direct access to physicians at the hospital or to MGH physicians by email, text, telephone, or video conference. We also made clear that DSC2U is not meant to help in emergencies or to address urgent medical issues.
Description of the PCP Plan
Our PCP WG was responsible for helping develop the content for the PCP Plan (Figure 2; Appendix Cii). The purpose of the PCP Plan was to provide specific recommendations for the PCP, based on the symptoms and concerns entered by the caregiver on the DSC2U intake. The recommendations on the PCP Plan (written in medical terminology) were consistent with those reported on the Caregiver Checklist (written in layperson terminology). The PCP WG worked with the expert advisory panel and our research team to develop a compact document intended to be a PCP-friendly companion document to the Caregiver Checklist. For example, if a caregiver indicated that the individual with DS had not had thyroid function tests performed in the past 12 months, the PCP Plan included a statement such as “According to the parent/caregiver, thyroid function tests have not been checked in the past 12 months. According to the national DS health care guidelines, we recommend that you consider ordering TSH and free T4 during today's visit.” (“T4” stands for “thyroxine”, and “TSH” stands for “thyroid-stimulating hormone”.) Our PCP WG emphasized that we needed to be mindful of the tone of our PCP Plans, as we wanted the community-based PCPs to view them as helpful rather than intrusive. Figure 2 displays an example of a PCP Plan that shows recommended laboratory tests and procedures, and potential new conditions to discuss with the patient. The laboratory tests include immunoglobulin A (IgA) total tissue transglutaminase (TTG)-IgA for a celiac screen, and TSH with free thyroxine (fT4) as a thyroid function test.

Figure 2
PCP Plan.
As drivers of the DSC2U intervention, we provided caregivers with both the Caregiver Checklist and PCP Plan. As part of the study protocol, we asked caregivers to share the PCP Plan with their child's or dependent's PCP at their upcoming visit. However, it is possible that the caregiver did not share the PCP Plan as designed.
In short, we developed the Caregiver Checklist and PCP Plans in such a way as to replicate—to the extent feasible—the medical information that we give to patients being seen, in person, at the MGH Down Syndrome Program. At all times, we encouraged caregivers to share the PCP Plan and to discuss the customized recommendations with their child's or dependent's PCPs.
Baseline Assessment
The content of the care received by both the intervention and control groups was ascertained through the Baseline Assessment (Appendix D) completed no more than 8 weeks before a wellness visit with the PCP. The date of each patient's PCP wellness visit was collected from the caregivers as part of the eligibility screening. To minimize loss to follow-up, reminder emails were sent 3 times about 2 weeks apart, concluding with 2 telephone calls in the eighth week by our research assistant.
The Baseline Assessment (Appendix D) requested the following information:
- Caregiver information: First name, last name, sex, date of birth, relationship to patient, phone number and address.
- Patient information: First name, last name, sex, date of birth, race/ethnicity (NIH standardized format), health insurance, education, marital status, health literacy, and numeracy.
- PCP information: First name, last name, sex, office phone number, office address and email (if available), and time to travel to PCP.
- Confirmation of the date of annual well visit (PCP visit) appointment: If this differed from that reported during the eligibility screening, we updated our records so that the timing of subsequent study questionnaires would be precise.
- Current symptoms, health history, and past medical history that would trigger recommendations for our 5 health care screenings (celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation): (See Appendix E for specific algorithms.) We assessed these symptoms among other symptoms not related to these conditions to minimize any priming effects. For example, “Does your child snore at night?” might be asked next to “Does your child have any rashes?” (A sleep study might be warranted for snoring, but not for rashes.)
- Recent completion of the following evaluations: celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation: These were collected to help determine patients' adherence to our primary outcome of 5 health screenings before randomization and intervention. We assessed these evaluations among other tests and procedures (eg, neck x-ray and dental visit) to minimize any priming effects.
- Secondary outcome measures assessing QOL: We used the Pediatric Quality of Life (PedsQL) 2.0 Family Impact Module (FIM) and PedsQL 4.0 parent-proxy, standard Short Form-15 (SF15) Generic Core Scales.
Study Outcomes
Primary Outcome
Specific aim 1 (primary outcome)
To test whether a customizable Caregiver Checklist and PCP Plan vs usual care would increase caregiver-reported, provider-driven health actions consistent with national guidelines.
As referenced in the Background section, the health care guidelines set forth by the AAP and expert consensus statements for DS define specific and measurable screening recommendations for individuals with DS.3-6 Our study was powered to detect improvement in adherence to 5 of these: celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation. We chose these 5 services because of the prevalence of the associated medical problems in people with DS, the consequences of failing to treat them appropriately, and the availability of treatments and therapies. Undiagnosed and untreated, these conditions directly affect cognitive development, school performance, QOL, and long-term morbidity. Every parent or caregiver we consulted for this project believed that these were important outcomes and were of paramount importance to the health and well-being of their sons or daughters.
Specifically, the guidelines are as follows:
- Celiac screen. If symptoms are present, obtain TTG-IgA and total IgA levels annually.
- Sleep study. Performed by 4 years of age and again if symptomatic.
- Thyroid test. TSH should be checked annually beginning at age 1 year.
- Audiogram. Annually up to age 21 years, and every 2 years thereafter.
- Ophthalmology evaluation. Ages 1 to 5 years, annually; ages 5 to 13 years, every 2 years; ages 13 to 21 years, every 3 years; and age 21 years and older, every 2 years.
We assessed the indications for 0, 1, 2, 3, 4, or 5 screenings for a given participant (see Appendix F for specific algorithms) based on age, biological sex, symptoms, and health history information reported in the Baseline Assessment (Appendix D) as described previously, in both the intervention and control groups.
Measuring outcomes related to health care
With direct input from our WGs, we developed the Health Care Outcome Survey (part of the 7-month follow-up survey) that caregivers completed after their child or dependent's PCP wellness visit (Appendix E). The purpose of this survey was to assess whether the primary outcome measure (ie, the 5 health screenings listed in the guidelines) for our study were recommended by the PCP and/or completed. These health care screenings were measured along with other health care outcomes to minimize response bias. The instructions clearly stated that the survey questions did not necessarily imply recommendations but instead measured various health care laboratory tests and procedures.
Secondary Outcomes
Specific aim 2 (secondary outcomes)
To determine whether a customizable Caregiver Checklist and PCP Plan vs usual care would be satisfactory to caregivers and PCPs and whether these customized plans would improve QOL for caregivers and families.
Measuring satisfaction with DSC2U
We wanted to determine whether using DSC2U was satisfactory to caregivers and providers. The Patient/Caregiver Experience Surveys (part of Appendix F) and PCP Experience Surveys (part of Appendix G) included 7-point Likert scale questions, multiple-choice options, and open-text responses solicited from caregivers and providers about their experience using DSC2U and their level of satisfaction with the content and guidance offered by the Caregiver Checklist and PCP Plan.
No existing validated surveys measure experience with DSC2U, but standard measures of patient experience are available and have been used with parents or caregivers of people with a range of abilities. Rather than assuming what questions should be asked to assess caregiver and PCP experience with DSC2U, our research team worked directly with our WGs to review established measures and develop new measures deemed meaningful to them. We relied as much as possible on previously validated tools and measures of patient experience in primary care settings.
Measuring QOL
We wanted to assess whether using DSC2U improved QOL for families over the full 10 months of the study as measured by the nationally validated PedsQL survey instruments.17-19 Our caregivers reviewed 2 validated surveys that we used for secondary measures: the PedsQL 2.0 FIM and PedsQL 4.0 parent-proxy, standard SF15 Generic Core Scales (https://www.pedsql.org/).17 The instruments—our QOL measures—were embedded within the Baseline Assessment (Appendix D), parent/caregiver 2-week follow-up survey (Appendix E), and 7-month follow-up survey (Appendix F).
We derived 6 summary scores from the 2 PedsQL instruments: measures of (1) psychosocial health, (2) physical health, and (3) an overall total score from the PedsQL 4.0 parent-proxy, standard SF15 Generic Core Scales; and measures of (4) parental health-related QOL, (5) family functioning, and an (6) overall total score from the PedsQL 2.0 FIM. We calculated all 6 summary scores according to the procedures recommended by the PedsQL developers.
Sample Size Calculations and Power
Preliminary Data
The PI for this trial had conducted a study on the number and type of evaluations required to comply with national recommendations in a sample of 103 patients with DS receiving usual care.8 The results indicated the following distribution of compliance with the 5 types of evaluations proposed as the primary outcome measure (for aim 1) for this trial: 9% with 0 evaluations, 18% with 1 evaluation, 22% with 2 evaluations, 27% with 3 evaluations, 15% with 4 evaluations, and 9% with 5 evaluations.8 In this previous sample, all 5 evaluations were indicated. This distribution has an SD of 1.4 evaluations and conforms closely to a β-binomial distribution with a mean of 2.5, the expected number of completed evaluations, and ρ of 0.15, the pairwise correlation among the 5 Bernoulli events for a given patient of having or not having each of the 5 recommended evaluations.
Minimum Effect Size
We powered our trial to detect an average treatment effect of 0.6 evaluations between the intervention and control groups after 7 months. This value is about 0.43 SD of the observed variation in screening evaluations by PCPs based on our preliminary data.8 Each one of these 5 evaluations is considered of paramount importance by the AAP and adult consensus statements in decreasing comorbidities. Further, our parent/caregiver WG reviewed with us these 5 evaluations and agreed that they were of critical importance to the health of their children with DS.
The proposed minimal treatment effect of interest of 0.6 evaluations would allow us to detect an improvement in the health actions for more than half of our population by at least 1 evaluation. This would be clinically meaningful for the DS community.
Power Calculations
The primary outcome follows a multinomial distribution, taking integer values from 0 through 5, the count of the number of recommended and indicated evaluations completed for a given patient with DS. Our preliminary data suggested that a β-binomial distribution matches the observed distribution well. The variance of a β-binomial taking values from 0 to n with mean μ and pairwise correlation ρ is μ (1 − μ/n) (1 + (n − 1) ρ).
The power for the primary analysis can be estimated from a 2-group t test with unequal variance and Satterthwaite df, controlled by a given difference in means. Assuming that all 5 evaluations are indicated for all participants, the mean number of evaluations among participants randomly assigned to usual care is 2.5. Moreover, pairwise correlation among evaluations is equal to 0.15, as we observed in our preliminary data. Thus, enrolling 200 total parents/caregivers and allowing up to a 14% dropout rate, we would have 80% power to detect an average increase of 0.6 evaluations completed by the PCP out of the 5 total recommended evaluations we proposed to track in this trial, which lasted for 10 months from the baseline assessment to the last survey completion (Figure 3).
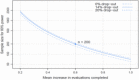
Figure 3
Sample Size Requirements for a Range of Effect Sizes and Dropout Rates.
A Monte Carlo simulation applying 2-group t tests to 10 000 sets of randomly generated β-binomial data with the specified parameters yielded 80% power when the true difference was 0.6 evaluations and a 5% type I error rate when the true difference was 0. The variance was maximal when we assumed that all 5 evaluations are indicated and at the observed mean of 2.5 evaluations. Thus, deviations from those assumptions in the study sample would result in increased power for detecting a true improvement of 0.6 evaluations.
Power for testing for subgroup differences in the efficacy of the Caregiver Checklist and PCP Plan intervention depends on the prevalence of specific subgroups of interest. Broadly, the study would have approximately 80% power for a 3-df test of age group–dependent differences in treatment efficacy if the age group × treatment interaction explained at least 6% of the variation in compliance with recommended evaluations.
We lacked preliminary data on person-to-person variation and covariance among repeated measures for our secondary outcomes. As a general guide, however, for a 2-group Wilcoxon rank sum test of normally distributed changes from baseline tested at a 2-tailed α = .008 to accommodate the 6 QOL measures and assuming up to 14% loss to follow-up, the study had 80% power to detect treatment-specific improvements (our secondary outcomes) with effect sizes (Cohen d) as small as 0.56.
Time Frame and Conduct of the Trial
Once we deemed a caregiver eligible according to the measures described in the “Participants” section, we allowed them to view our online consent form. As part of the participant's consent process, we provided them with information about being randomly assigned to either group A or group B, as shown in Figure 4. This included written assurance that both groups would get access to DSC2U during the trial (for group B, this would be at the end of the study). All participants were asked to indicate that they understood the information provided and consented to their full randomized participation.
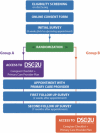
Figure 4
Study Procedure for 2-Group RCT of DSC2U.
Once they had provided consent, study personnel were notified via email, and the Baseline Assessment (Appendix D) was sent electronically to the participant for completion no more than 8 weeks before the scheduled PCP visit. If the survey was not completed, a reminder email was sent every 2 weeks over the course of a 6-week period. If still not completed after 6 weeks (3 email reminders later), we made up to 2 telephone calls to ask for completion of the survey.
Once participants had completed the Baseline Assessment (Appendix D), we randomly assigned them 1:1 to either the intervention or the control group, as shown in the top part of Figure 4 through the green box. Participants were assigned in a 1:1 ratio to DSC2U or a waitlist, according to a computer-generated randomization schedule constructed with permuted blocks of sizes 2 and 4, stratified for distance from their PCP (3 levels: <30 minutes, 30-59 minutes, and ≥60 minutes) and type of insurance (2 levels, public and private).
The bottom half of Figure 4 shows what the intervention group (group A) and the control group (group B) experienced in the main part of the trial. For group A, after the caregivers submitted the Baseline Assessment (Appendix D), we emailed them a link to DSC2U (purple box); they could access it with a 4-digit passcode so that they could return to and complete the DSC2U form at their convenience. If any participants did not complete the DSC2U, we emailed reminders to complete the form at 4, 3, and 2 weeks before their child's or dependent's scheduled PCP appointment. After they completed the DSC2U intake questionnaire, the system immediately sent the participant their personalized Caregiver Checklist and PCP Plan; these were accessible in their DSC2U portal, accessible only with the passcode. The Caregiver Checklist and PCP Plan could be viewed, printed, or emailed to themselves or others at the user's discretion.
After submitting the Baseline Assessment (Appendix D), participants in group B were sent an email message thanking them for their participation in the study. Included was a reminder to let study personnel know if their child's or dependent's scheduled appointment with the PCP had changed.
Figure 5 depicts the steps for communicating with the participants and gathering data from the various surveys described earlier. Specifically, a maximum of 2 months elapsed between the Baseline Assessment (Appendix D) and each patient's scheduled visit with the PCP (time T0).
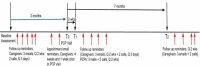
Figure 5
Survey Implementation at Various Time Points.
Before each patient's scheduled PCP visit, we sent the caregiver 2 reminders for their upcoming appointment. This included a request to let our study personnel know whether the date of the appointment had changed. Because the timing between study enrollment and the scheduled PCP visit naturally varied among participants, we sent these appointment reminders at approximately 4 weeks and then 1 week ahead of the scheduled appointment.
The Parent/Caregiver Experience Surveys (part of Appendix F) and PCP Experience Surveys (part of Appendix G) were administered approximately 2 weeks after the PCP visit as part of the 2-week follow-up survey (see Figure 5, T1). This allowed caregivers and PCPs to provide feedback while the office visit was still fresh in their minds. Caregivers were invited by email to complete their Patient/Caregiver Experience Surveys (part of Appendix F). PCPs received their PCP Experience Surveys (part of Appendix G) by mail, with an option to complete the survey electronically. PCPs whose email addresses had been provided by the caregivers also received a direct invitation by email to complete their survey. To minimize loss to follow-up, reminder emails were sent 3 times about 2 weeks apart, concluding with 2 telephone calls in the eighth week by our research assistant.
We asked caregivers by email to complete the 7-month follow-up survey after the PCP visit (Figure 5, T2). This allowed the maximum time within the constraints of the grant's timeline to measure whether the recommended health care actions, as mentioned on the personalized Caregiver Checklists and PCP Plans, had been implemented. (Some of the health care recommendations, such as getting a sleep study, can take up to 5 or 6 months to be scheduled and ordered in some parts of the country.) Again, to minimize loss to follow-up, reminder emails were sent 3 times about 2 weeks apart, concluding with 2 telephone calls in the eighth week by our research assistant.
Data Collection and Sources
Data Supporting Primary Outcome
Primary outcome measures were collected from the caregiver-completed 7-month follow-up survey. We chose to measure the primary outcomes from caregivers, rather than from PCPs, for several reasons. Caregivers were more likely to be invested in the project and have time to respond. In addition, we expected them to be more knowledgeable of the results. For example, a child or dependent might have seen an ophthalmologist, upon referral from the PCP, but the caregiver, rather than the PCP, would know if this had happened.
To validate caregiver responses, we verified the documentation obtained from 20% of the participants. For this validation, we sought permission from the caregivers to request medical records from their child's or dependent's PCP. Based on the records obtained, we assessed whether each of the 5 evaluations associated with our primary outcome had been performed, as reported in the 7-month follow-up survey, completed by caregivers, and administered 7 months after a wellness visit with the PCP. For example, if a caregiver reported that a sleep study had been done, we sought medical documentation indicating that such an evaluation had occurred. This type of patient health-related data was scanned and stored electronically in our secure, access-limited, password-protected research folder on our hospital's HIPAA-compliant servers.
Secondary outcome measures related to satisfaction with DSC2U were measured for both caregivers and PCPs; those related to QOL were measured only for caregivers. In addition to the data collected to support our primary and secondary outcomes, we also measured the caregiver‘s experience of the PCP visit using standard measures such as the duration of the patient-provider relationship, quality of communication, and overall visit experience using measures taken or adapted from the Clinician & Group Survey Consumer Assessment of Healthcare Providers and Systems (CG-CAHPS) suite of instruments (https://www.ahrq.gov/cahps/surveys-guidance/cg/index.html). We wanted to use standard patient experience measures that were visit specific and selected measures from the CG-CAHPS. CAHPS tools are required for public reporting by the Centers for Medicare & Medicaid Services and have been tested extensively and disseminated widely for use in primary care for adults, children, and their proxy respondents. Additionally, we asked PCPs for information about themselves (degree, specialty, race/ethnicity), about their practice (number of PCPs in the practice, the practice location type, and whether they were working in a federally qualified community health center), and about how difficult ordering tests or making referrals is for them.
Four randomly assigned participants withdrew from the study on their own account. We ascertained the reasons through email correspondence. One person enrolled in a DS specialty clinic and self-withdrew. Another was uncomfortable with the electronic transmission of information. A third family moved to another state and was not able to establish a new PCP within the study time frame. The fourth person needed to reschedule the PCP date outside our study window. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Partners HealthCare.20-22
REDCap (https://www.project-redcap.org/) is a secure, web-based software platform designed to support data capture for research studies. It provides (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources. The data that we collected through REDCap are secured and stored behind our hospital's HIPAA-compliant firewall and follow Partners HealthCare Information Security policies for authenticated, minimum access.
Analytical and Statistical Approaches
Our primary analysis compared the mean number of indicated evaluations that parents or caregivers reported had been recommended or completed within 7 months of the index PCP visit between the Caregiver Checklist and PCP Plan intervention and usual care groups. For participants lost to follow-up and those reporting that they were not sure whether a given evaluation item was completed or recommended by their PCP, we counted them as having not completed the evaluation item. (In this case, we are treating loss to follow-up as fully informative about noncompletion.) On the other extreme, as part of our Supplementary Materials (Appendix H), we performed the same analyses but excluded any missing data. (In this case, we are treating loss to follow-up as wholly noninformative [ie, completely at random] with respect to noncompletion.) We analyzed the data using a 2-sample t test. We confirmed our inference using a generalized linear model assuming β-binomial–distributed counts of indicated evaluations conditional on treatment group assignment, a logit link with parameters estimated by maximum likelihood, and a permutation test by direct randomization of assignment to control or intervention groups. Exact unconditional 95% CIs on reported rate ratios and risk differences were calculated by inverting separate 1-sided exact tests of the score statistic.22
We declared the benefit of the intervention significant if the mean number of indicated evaluations that had been recommended or completed was greater among participants randomly assigned to the Caregiver Checklist and PCP Plan intervention and if a 2-tailed P value for the treatment comparison was <.05. We concluded that the effect of the intervention was statistically significant only if the permutation test concurred with inference from the β-binomial regression, as the permutation test makes no assumptions concerning the true distribution of the data.
We analyzed longitudinal changes in our secondary outcome measures using a shared-baseline repeated-measures analysis of variance for each of 6 QOL outcomes derived from the 2 PedsQL instruments. Linear contrasts were used to test for treatment-specific improvements over time. As a sensitivity analysis, we also used simple 2-group Wilcoxon rank sum tests to compare changes from baseline in each follow-up evaluation individually. With 6 secondary outcomes, we tested each secondary outcome at a 2-tailed α = .008 to control for multiple comparisons.
We analyzed the primary outcome for subgroup-dependent differences in the efficacy of DSC2U in linear models that included terms for subgroup membership, treatment group (intervention vs control), and their interaction and that allowed for heteroscedastic variance by subgroup. Subgroups were prespecified by the following attributes: (1) giving or not giving the PCP Plan before or during the wellness visit; (2) completing or not completing the 2-week and 7-month surveys after the PCP visit; (3) race and ethnicity, comparing White non-Hispanic individuals with DS with all others with DS; (4) the age of individuals with DS >18 years vs ≤18 years; and (5) having private vs public insurance coverage. Subgroup-specific estimates were obtained by contrasts of least-square means. Evidence of a subgroup-dependent difference in the efficacy of DSC2U was based on the significance of the subgroup × treatment group interaction.
We evaluated the predictors of the primary end point and several ordinal measures of participant-reported evaluations of the Caregiver Checklist and PCP Plan with Spearman rank correlation and in a series of univariate ordinal logistic regression models using cumulative logits.
Changes to the Original Study Protocol
We made a few changes from our original study protocol, which were approved by the MGH IRB. First, to reduce the impact of loss to follow-up on power, we implemented 2 strategies: (1) We increased the enrollment number from 200 to 230, and (2) we added 2 contact methods (postal mail and/or a telephone call) for study participants who did not complete the 2-week survey and/or the 7-month survey. Second, we added a DSC2U form click-tracking method to understand the use of informational resources in the Caregiver Checklist and PCP Plan. Third, we provided an optional user experience REDCap survey to all participants in the trial's control group at the end of the study to solicit comments and suggestions on improvements to the Caregiver Checklist and PCP Plan. Last, all DSC2U stakeholders (parents/caregivers WG, PCP WG, expert advisory panel, and the key personnel) were invited to complete the PEIRS, a survey developed by a team in Canada, which we formatted into REDCap to assess the quality of engagement and to solicit constructive feedback on ways to strengthen our key stakeholder engagement in future studies. The results of this survey are reported in Table 4.
Results
Participant Flow
From October 3, 2017, to September 30, 2018, we assessed 645 caregivers for eligibility through the study website, and 281 caregivers were consented (Figure 6). After accounting for the caregivers who did not complete the baseline survey, changed their PCP visit date outside the study window, or self-withdrew, we randomly assigned 230 consented caregivers to receive either DSC2U (n = 117 participants) or usual care (n = 113 participants). Usual care was defined as the routine care that patients would normally receive through their PCP.
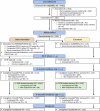
Figure 6
CONSORT Diagram.
The CONSORT diagram in Figure 6 shows the enrollment of participants (both PCPs and caregivers), their allocation of treatment, their follow-up surveys (both 2-week follow-up survey and 7-month follow-up survey), and analysis.
Two weeks after the PCP visit, 101 of 113 (89.4%) caregivers who had been assigned to the intervention group completed the study survey. The unadjusted rate among all 117 originally assigned to the intervention group was 84%. In the control group, 108 of 113 eligible (96%) caregivers completed this same survey. At this same time point, we surveyed PCPs. In the intervention group, we received completed surveys from 82% of the PCPs in the intervention group and 78% of the PCPs in the control group.
Seven months after the PCP visit, we received surveys from some participants who had not responded at the 2-week time point. The response rates were 90% and 97% from caregivers in the intervention group and control group, respectively.
Baseline Characteristics of the Intervention and Control Groups
The ages of the individuals with DS ranged from 8 months to 56 years of age (at the time of the caregivers' completion of the Baseline Assessment [Appendix D]); 92% of the caregivers were mothers. The 2 groups comprising caregivers, patients, and PCPs (ie, intervention and control groups) were well balanced with respect to baseline characteristics and relationships, as would be expected from randomization. (For details, please see Tables 6A-8B.)
Table 6A
Baseline Characteristics of People With DS.
Table 6B
Baseline Characteristics of Parents or Caregivers for Persons With DS.
Table 6C
Baseline Characteristics of PCPs.
Table 7
Practice Characteristics of the PCPs at Baseline.
Some small differences emerged because of chance. For example, more participants in the DSC2U treatment group than in the usual care group reported seeing the same PCP for ≥5 years; fewer participants in the intervention group reported seeing their PCP for <6 months (Table 8A). PCPs were also more likely to feel that caregivers in the intervention group were definitely able to provide information that the PCP needed to assess the patient's history and to create a care plan (Table 8B). No other differences were notable.
Table 8A
Relationship Between Parent or Caregiver and PCP at Baseline.
Table 8B
Relationship Between PCP and Patient or Caregiver (or Both) at Baseline.
Primary Outcome
Specific aim 1 (primary outcome): To test whether a customizable Caregiver Checklist and PCP Plan vs usual care would increase caregiver-reported, provider-driven health actions consistent with national guidelines.
Our study was powered to detect improvement in adherence to 5 of the screening recommendations set forth by the AAP and adult consensus statements for DS: celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation. (See Tables 9A-9C and Appendix H, Supplementary Materials S12: Tables S3a-3c for details.) Relative to participants receiving usual care, participants in the intervention group had a 1.6-fold increase in the number of indicated evaluations that were completed or recommended by the PCP. The absolute difference was 0.20 evaluations between the intervention group and the control group (Table 9C). (See Appendix H, Supplementary Materials S12: Tables S3a-S3c for modified Tables 9A-9C, where missing data were instead treated as wholly noninformative—ie, completely at random—with respect to noncompletion.)
Table 9C
Primary Outcome: Indication and Completion of Total Evaluations (Missing Evaluations Treated as Noncompletion).
Table 9B
Primary Outcome: Completion of Indicated Assessments (Missing Evaluations Treated as Noncompletion).
Taken individually, the audiograms were statistically more likely to be completed or recommended by the PCP in the intervention group than in the usual care group. Roughly twice the proportion of participants in the intervention group had indicated celiac and sleep study evaluations that were recommended or completed, but these comparisons did not reach statistical significance (Table 9A). Of note, the intervention group was also more likely to complete nonindicated evaluations than was the control group (Appendix H, Supplementary Materials S12), although the difference was not significant when classifying loss to follow-up as noncompletion.
Table 9A
Indicated and Completed Individual Evaluations (Missing Evaluations Treated as Noncompletion).
Treatment Response Heterogeneity
We performed separate analyses of our primary outcome measures on various subgroups. (Data are reported in Appendix H, Supplemental Materials S13 Tables S3a through S8b.) Through this analysis, we wanted to see whether specific subpopulations of our participants were more likely to benefit from DSC2U than were others. We compared participants who used the intervention as it had been designed—specifically that they would share the DSC2U tool with the PCP before or during the scheduled visit—with those who did not (Appendix H, Supplemental Materials S13, Tables S4a and S4b). We also compared those who completed all study evaluations—specifically, both the 2-week and 7-month surveys—with those who did not. Regarding sociodemographic or clinical factors, we compared persons with DS who were non-Hispanic White with those of other races and ethnicities, those persons with DS who were adults (>18 years) with younger patients, and persons with DS who had private insurance with those with public coverage or self-pay (Appendix H, Supplemental Materials S13 Tables S5a through S8b). None of these subgroups disproportionately benefited from DSC2U based on our primary outcome measure.
Validation of Caregiver-Reported Health Outcomes
We validated the reports of 5 primary outcome measures from 20 caregivers. Of these 100 parental reports, 95 reports were accurate based on medical records; we received 5 inconsistent responses from 4 caregivers. In short, caregivers were highly accurate historians in reporting on the measured outcomes.
Secondary Outcomes
Specific aim 2 (secondary outcomes): To determine whether a customizable Caregiver Checklist and PCP Plan vs usual care would be satisfactory to caregivers and PCPs and could improve QOL for caregivers and families.
Satisfaction With DSC2U
Caregivers reported high satisfaction with DSC2U at both the 2-week time point (mean [SD], 7.75 [1.84]; scale, 0-10) and 7-month time point (mean [SD], 7.79 [1.82]); satisfaction exhibited no significant decline over this time (P = .895, Tables 10A and 10B). PCPs also expressed high satisfaction with DSC2U at the 2-week time point (mean [SD], 7.80 [1.86]; scale, 0-10).
Table 10A
Secondary Outcomes: DSC2U Experience Measures for Parent or Caregiver.
Table 10B
Secondary Outcomes: DSC2U Experiences Measures for PCP.
Quality of Life
Analyses of secondary QOL outcomes, as ascertained through the 6 PedsQL 4.0 parent-proxy and PedsQL 2.0 FIM Summary Scores, did not demonstrate a significant difference between the intervention and control groups at either the 2-week or 7-month time points (Table 11).
Table 11
Secondary Outcomes: Change From Baseline on QOL Measures.
Ancillary Analyses
The additional, prespecified ancillary analyses were focused on the experience surveys and regression analyses. They were planned (1) to further investigate the caregiver and PCP experience with using DSC2U and (2) to determine, through regression analyses, whether any demographic characteristics could predict our primary and secondary outcome measures.
Caregiver and PCP Experience Using the DSC2U
Of the caregivers in the intervention group who completed the 2-week follow-up survey (n = 101), all accessed the Caregiver Checklist in some capacity (viewing, downloading, and/or printing). Of these, 97% reported that the Caregiver Checklist recommendations were easy to understand (Table 10A). All caregivers would recommend the Caregiver Checklist to another caregiver. About 76% would review their Caregiver Checklist more than twice per year, and 96% of caregivers would complete DSC2U again in the future (Table 10A).
About 60% of caregivers in the intervention group shared the PCP Plan with their PCPs before or during the visit, and 4 shared the PCP Plan afterwards (Table 10A). Of the 36 caregivers who did not share a copy of the PCP Plan with their PCP, most either chose not to (n = 8) or forgot to (n = 10). When the PCP Plan was shared with the PCP, 93% of caregivers felt that the PCP seemed to be interested in the information, and 86% felt that the PCPs were in agreement with the recommendations.
Of the 94 PCPs who completed their 2-week follow-up survey, about 40% reported being given the PCP Plan at or before the visit (Table 10B). Of these PCPs, all discussed the PCP Plan with the caregiver and said in study surveys that they were interested in the information. About 97% agreed with the recommendations in the PCP Plan.
Regression Analyses
We used simple rank correlations and a series of univariate ordinal logistic regression analyses to test whether certain characteristics of the individual with DS, the caregiver, the PCP, the PCP's practice, or the relationship between the PCP and caregiver (ie, the duration of relationship, quality of communication, and overall visit experience) significantly predicted the number of indicated evaluations that were completed or recommended. We also used the same set of independent variables to predict the following dependent variables: how the caregiver would rate the Caregiver Checklist, whether the caregiver would recommend DSC2U to another caregiver, whether the caregiver would re-read or reuse links in the Caregiver Checklist, and whether the caregiver would complete the DSC2U intake form again. With respect to PCPs, we again used the same independent variables to predict whether the PCP discussed the PCP Plan or any of its recommendations with the caregiver, whether the PCP was interested in any of the information in the PCP Plan, whether the PCP agreed with the recommendations in the PCP Plan, and how the PCP would rate the PCP Plan.
None of the characteristics of the individual with DS, the caregiver, the PCP, the PCP's practice, or the relationship between the PCP and caregiver were statistically significant predictors for any of the dependent variables detailed above when the P value was adjusted to account for multiple comparisons (Appendix H, Supplemental Table S9). Given the lack of univariate association, we did not investigate these models further.
Discussion
Principal Findings
Our national, 2-group, RCT had 2 main aims. Aim 1, our primary outcome, focused on the impact of a unique online tool, DSC2U, on adherence to 5 important health screenings for patients with conditions related to their DS. Aim 2, our secondary outcomes, examined the satisfaction of caregivers with use of the DSC2U and its perceived impact on QOL for their families. DSC2U, a novel health care tool, produced a customized (individualized) Caregiver Checklist and a PCP Plan that caregivers could share with the PCP providing care to their family member with DS.
Overall, the trial findings suggest that health care guideline adherence of individuals with DS can be improved when caregivers are empowered with up-to-date, patient-specific, curated medical information. We further demonstrated that PCPs are both eager to receive and willing to recommend such customized suggestions.
Receipt of Crucial Screening for Important Coexisting Conditions
Patients with DS whose caregivers (defined as parents, siblings, or other persons responsible for the care of an individual with DS whom they identify as a “dependent”) accessed DSC2U were more likely to complete or have their PCPs recommend 5 nationally recommended health care evaluations for DS: a celiac screen, sleep study, thyroid test, audiogram, and ophthalmology evaluation. The number of indicated evaluations that were completed or that the PCP recommended was 1.6-fold greater among families offered the use of DSC2U than among those receiving standard of care. The mean absolute difference was 0.20 evaluations across the 5 evaluation items between the intervention group and the control group. Put another way, there was 1 more indicated evaluation that was recommended or completed for the intervention group than for the control group for every 5 PCP visits.
To our knowledge, DSC2U is the first online tool directed toward the caregiver community that not only has been created to support the wellness of individuals with DS but is also known to be effective in improving their health-related outcomes. More than 95% of individuals with DS in the United States lack access to a DS-specific clinic. This outcome will be a welcome one: DSC2U was effective in bringing high-cost specialty-level care to lower-cost primary care settings.
Satisfaction With the Online Tool, Caregiver Checklist, and PCP Plans
Caregivers and PCPs were also highly satisfied with the tool. The majority of caregivers indicated that they would revisit their Caregiver Checklist more than twice a year. Nearly all reported that they would re-complete the DSC2U at least once a year. Nearly all PCPs agreed with the recommendations in the PCP Plans.
These results were particularly notable because only 60% of caregivers randomly assigned to the intervention group reported that they had shared the PCP Plan with the PCPs. Moreover, only 40% of the PCPs remembered receiving the PCP Plan before or after the annual wellness visit. The reasons for the discrepant responses between caregivers and PCPs are not immediately clear. They might include overreporting by caregivers perhaps embarrassed to acknowledge protocol noncompliance, poorer recall by PCPs who are caring for multiple patients, or some combination of such factors. Regardless, our trial still achieved a positive primary outcome—a result even more meaningful when considering the likelihood that, in the real-world application of DSC2U, many caregivers might forget or choose not to share the PCP Plan with PCPs. An open question remains: Could the impact of DSC2U have been even greater if more caregivers had shared the PCP Plans with the PCPs?
QOL Outcomes
During the trial, caregivers who used DSC2U did not report a significant improvement in QOL for the individual with DS or for their families. A reason for this could be that the 7-month follow-up time frame was too short for caregivers to appreciate a change in QOL. Many families could have been experiencing stressors that often accompany new diagnoses (eg, celiac disease). More time might have been needed (eg, becoming accustomed to a sleep mask) before the benefits of treating a new disease (eg, obstructive sleep apnea) were realized. Alternatively, the patients with DS might have already been receiving good (although not ideal) health care from their PCPs at the beginning of the study. This might have made it harder to appreciate a more positive change. Last, the QOL survey instruments had been previously validated on a neurotypical population. The measures were not specific to the DS population, and therefore may not have captured actual QOL changes.
Treatment Response Heterogeneity
Our subgroup analyses included examining people with DS by race, ethnicity, age, and insurance type. These analyses did not demonstrate differences in our primary outcome. Further, our regression analyses did not uncover certain patient, caregiver, or PCP characteristics that could predict the use or relevance of DSC2U (ie, whether the caregiver would recommend DSC2U to another caregiver, whether the caregiver would re-read or reuse links in the Caregiver Checklist, whether the PCP agreed with the recommendations in the PCP Plan, and how the PCP would rate the PCP Plan).
This trial, however, was not powered for these secondary or subgroup analyses. We conclude that further research is needed to determine whether effect size and satisfaction levels vary among subgroups within the DS community.
Study Limitations
Our trial was not without limitations, the first of which included sampling bias. Our caregiver cohort was largely White and well educated: 73% had a 4-year college degree or higher, and only 11% scored low on our health literacy assessment. They were also highly capable and invested in the wellness of the individual with DS. For example, >90% of PCPs “definitely” felt that the caregiver for the individual with DS was able to provide the information needed during wellness visits. Also, most of these patients were well cared for at baseline; they only had 1 or 2 evaluations missing out of 5.
Caregivers who participated were also eager (perhaps more so than those who did not volunteer) for this type of research, as evidenced by the speed of our recruitment. Within 30 hours of a single post on social media by the PI, our study team had recruited the targeted goal of non-Hispanic White caregivers. After enrollment, these caregivers were also extraordinarily engaged, as evidenced by the high completion rates of all study surveys throughout the study. Overall, the participating caregivers were largely well educated, well resourced, and eager to access health information; to this extent, our results might not be generalizable to all families who have children with DS.
The PCPs also had a high response rate, with minimal follow-up effort needed from our study staff. This level of provider engagement might suggest that we sampled a very invested group of PCPs, who might also not be generalizable to all primary care communities.
Our study was also subject to differential recall bias. Caregivers in the intervention group may have been more likely than those in the control group to recall whether health evaluations were completed or recommended by the PCP. Caregivers in the intervention group may have paid closer attention to their dependent's medical care simply by participating in DSC2U. Moreover, any method of increasing awareness of DS health guidelines might have had a similar effect. In a future study, we would include a third group that would offer caregivers access to a nonpersonalized version of the Caregiver Checklist and PCP Plan that addressed the DS health care guidelines in a general way. Such a design would help us understand the incremental benefit of offering the automatically generated, personalized advice central to DSC2U.
We know that DS naturally occurs equally in all races and ethnicities, regardless of socioeconomic status. Although we were able to meet our enrollment target for Hispanics/Latinos, we were unable to achieve our targeted goal for Black participants. We recruited over 9 months through (a) a Black caregiver who served on our caregiver WG, providing monthly advice; (b) our recruitment materials and webpage, as they featured children with DS who were Black; (c) ethnically and racially diverse working groups from national DS organizations; (d) the local DS support groups in demographic areas of the country that have sizable Black communities; and (e) an African American caregiver who is an influencer on social media.
These efforts were not enough; however, we gained valuable insights about members of the Black DS community through feedback gathered from some caregiver WG members. We learned that some members expressed a distrust of research in general, based on the historic mistreatment of Black people in clinical research (eg, the Tuskegee syphilis experiment). Some members apparently believed that everything is “just fine” for their child with DS and did not want to “rock the boat,” even if they might be lacking in health care maintenance. Alternatively, some people had firm religious convictions that God would take care of their son or daughter and that medical follow-ups were unnecessary. Last, we learned that access to medical care can be expensive and cost prohibitive, particularly for families that have multiple financial demands. To this extent, our results might not be generalizable to the Black/African American DS community. Future research is needed to learn how to disseminate and implement DSC2U within this community.
Similarly, our study team recruited only 2 Spanish-speaking caregivers, even though our study materials were translated into and available in Spanish. One of our caregiver advisors, who is a DS advocate in the Latino community, shared the view that DSC2U offers great promise to families with a lower socioeconomic standing and a limited proficiency in English. She thought, however, that participation in all the elements of our research might have been too great a barrier to potential participants. We are interested to learn how to better disseminate and implement DSC2U to Spanish-speaking populations in the United States. An empowering caregiver suggestion is to connect caregiver advocates in this community.
The number of indicated evaluations that were completed or that the PCP recommended was 1.6-fold greater among families offered the use of DSC2U than among those receiving standard care. The absolute difference was 0.20 evaluations between the intervention group and the control group. For our power calculations, we had specified a priori that a difference of 0.6 evaluations was the minimum treatment effect of interest. Our consideration of an absolute difference of 0.6 evaluations at the time the study was designed was informed by our preliminary data, which indicated that individuals with DS were typically deficient on 2.5 evaluations. A potential “relative effect” of DSC2U can be as clinically important as an “absolute effect.” Within our population that was deficient on 1.5 evaluations, on average, an increase in the recommendation or completion of 0.20 indicated evaluations, on average, is a meaningful benefit to this population. These evaluations are deemed to be of clinical importance by the AAP and other guidelines.7,8
The funding from this grant allowed us time to assess adherence to the indicated evaluations up to 7 months after the wellness visit with the PCP. With more follow-up time, more indicated evaluations might have been completed. With the randomized design of our study, however, we would expect this impact to be the same for both the intervention and control groups, and more indicated evaluations would not have changed the overall conclusions of this study.
We also discovered that, compared with controls, patients in the intervention group were more likely to complete nonindicated evaluations, although the difference was not significant when classifying loss to follow-up as noncompletion. Receiving the PCP Plan may have caused PCPs in the intervention group to pay closer attention to the care of these patients. When examined individually, 2 of these screenings (celiac and thyroid screening) were statistically more likely to be completed, or at least recommended, when not indicated in the intervention group. These blood tests may have been easier to obtain, or their clinical triggers might have been more likely to intersect with neurotypical health care. Sleep studies, ophthalmology exams, and audiograms did not seem to be obtained as much when they were not clinically indicated. Notably, the Caregiver Checklists and PCP Plans did not enumerate which evaluations were not needed; only actions on missing items were suggested. To reduce health care costs even further, future versions of DSC2U might consider explicitly listing evaluations that are not clinically indicated.
Future Research
The results of this study demonstrate clear promise of a novel technology that not only addresses disparities in health care but also highlights how caregivers can be empowered as change agents. Whether DSC2U could be equally effective within more diverse communities, including Black families, families who speak Spanish as their preferred language, and families lower socioeconomic means, is of interest. We had a lower-than-expected representation of these groups in the current study.
Although our trial focused specifically on the basic health care recommendations for patients with DS, an important topic for follow-on investigations might be to extend the DSC2U support model to address other health issues that disproportionately affect individuals with DS. One such condition warranting further study is dementia.
Finally, we hope that DSC2U can serve as a blueprint for many other health care conditions beyond DS. The ingredients that we believe were crucial to the success of DSC2U included building a care delivery model centered around an invested caregiver (or patient); delivering curated, up-to-date medical information in a way that is meaningful to them; and incorporating evidence-based national guidelines and expert consensus into easily accessed materials. These components could also apply to many other medical conditions, covering a range of rare to relatively common conditions in both pediatric and adult populations, such as Marfan syndrome, 22q11 deletion syndrome, Turner syndrome, adult congenital heart disease, and pediatric food allergies. Adapting DSC2U for these conditions has the potential to be “game changing” for well-intentioned modern-day PCPs who are increasingly tasked with complex health care recommendations for a growing number of conditions.
Conclusions
DSC2U, a novel web-based tool for caregivers of people with DS, was created to provide up-to-date, personalized recommendations for people with DS. DSC2U improved adherence to national DS health care guidelines with patient-specific content that was highly appreciated by both caregivers and PCPs. Our positive results demonstrated that our novel technology, which empowered caregivers with evidence-based medical information, has the potential to transform how high-cost specialty care can be disseminated into low-cost primary care settings.
Footnotes
- i
Appendices B and C are a simulated version of the materials and do not describe a real patient.
- ii
Appendices B and C are a simulated version of the materials and do not describe a real patient.
References
- 1.
- de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2016;19(4):437-447. doi:10.1038/gim.2016.127 [PubMed: 27608174] [CrossRef]
- 2.
- de Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am J Med Genet A. 2015;167A(4):756-767. doi:10.1002/ajmg.a.37001 [PubMed: 25822844] [CrossRef]
- 3.
- Bull MJ, Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393-406. doi:10.1542/peds.2011-1605 [PubMed: 21788214] [CrossRef]
- 4.
- Chicoine B, McGuire D. The Guide to Good Health for Teens & Adults With Down Syndrome. Woodbine House; 2010.
- 5.
- Steingass KJ, Chicoine B, McGuire D, Roizen NJ. Developmental disabilities grown up: Down syndrome. J Dev Behav Pediatr. 2011;32(7):548-558. doi:10.1097/DBP.0b013e31822182e0 [PubMed: 21743353] [CrossRef]
- 6.
- Smith DS. Health care management of adults with Down syndrome. Am Fam Physician. 2001;64(6):1031-1038. [PubMed: 11578024]
- 7.
- O'Neill ME, Ryan A, Kwon S, Binns HJ. Evaluation of pediatrician adherence to the American Academy of Pediatrics health supervision guidelines for Down syndrome. Am J Intellect Dev Disabil. 2018;123(5):387-398. doi:10.1352/1944-7558-123.5.387 [PubMed: 30198765] [CrossRef]
- 8.
- Skotko BG, Davidson EJ, Weintraub GS. Contributions of a specialty clinic for children and adolescents with Down syndrome. Am J Med Genet A. 2013;161A(3):430-437. doi:10.1002/ajmg.a.35795 [PubMed: 23401090] [CrossRef]
- 9.
- Santoro SL, Martin LJ, Pleatman SI, Hopkin RJ. Stakeholder buy-in and physician education improve adherence to guidelines for Down syndrome. J Pediatr. 2016;171:262-268.e2. doi:10.1016/j.jpeds.2015.12.026 [PubMed: 26831529] [CrossRef]
- 10.
- Santoro SL, Bartman T, Cua CL, Lemle S, Skotko BG. Use of electronic health record integration for Down syndrome guidelines. Pediatrics. 2018;142(3):e20174119. doi:10.1542/peds.2017-4119 [PubMed: 30154119] [CrossRef]
- 11.
- Global Down Syndrome Foundation. Down syndrome medical care centers. Published May 2013. Accessed October 23, 2019. https://www
.globaldownsyndrome .org/research-medical-care /medical-care-providers/ - 12.
- National Down Syndrome Society. Down syndrome clinics and health care providers database. Published 2012. Accessed October 6, 2020. https://www
.ndss.org /resources/healthcare-providers/ - 13.
- Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ. 2007;335(7609):24-27. [PMC free article: PMC1910640] [PubMed: 17615222]
- 14.
- Murray E, Burns J, See TS, Lai R, Nazareth I. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;(4):CD004274. doi:10.1002/14651858.CD004274.pub4 [PubMed: 16235356] [CrossRef]
- 15.
- Hasman L, Zafron M. An analysis of online resources for parents, siblings, and other caregivers of adults with intellectual disabilities. J Consum Health Internet. 2010;14(1):33-41.
- 16.
- Hamilton CB, Hoens AM, McQuitty S, et al. Development and pre-testing of the Patient Engagement In Research Scale (PEIRS) to assess the quality of engagement from a patient perspective. PLoS One. 2018;13(11):e0206588. doi:10.1371/journal.pone.0206588 [PMC free article: PMC6211727] [PubMed: 30383823] [CrossRef]
- 17.
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126-139. doi:10.1097/00005650-199902000-00003 [PubMed: 10024117] [CrossRef]
- 18.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in healthy and patient populations. Med Care. 2001;39(8):800-812. doi:10.1097/00005650-200108000-00006 [PubMed: 11468499] [CrossRef]
- 19.
- Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL Family Impact Module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2(1):55. doi:10.1186/1477-7525-2-55 [PMC free article: PMC521692] [PubMed: 15450120] [CrossRef]
- 20.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010 [PMC free article: PMC2700030] [PubMed: 18929686] [CrossRef]
- 21.
- Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208 [PMC free article: PMC7254481] [PubMed: 31078660] [CrossRef]
- 22.
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213-226. doi:10.1002/sim.4780040211 [PubMed: 4023479] [CrossRef]
Acknowledgments
The authors acknowledge the contribution of DS-Connect (The Down Syndrome Registry), which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, for the data, study recruitment, and other assets used in this report.
We are also thankful to Caitlin Woglom for providing insight into DSC2U's nutrition-based questions and to Maggie Balz for providing insight into DSC2U's swallowing-based questions. We are grateful to Beth Watters, Rosemary Guiltinan, and Elizabeth Azano of Partners Office of General Counsel for their legal consultation on our description of DSC2U and the customized outputs. Ye Chin Lee of the MGH Laboratory of Computer Science provided valuable advice on the long-term sustainability of DSC2U. We especially thank all of the DS nonprofit organizations that advertised our trial to their members.
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#AD-1507-31567). Further information available at: https://www.pcori.org/research-results/2016/does-web-based-platform-caregivers-help-people-down-syndrome-get-recommended
Appendices
Appendix A.
Creation of DSC2U (PDF, 602K)
Appendix B.
Caregiver Checklist (PDF, 207K)
Appendix C.
PCP Plan (PDF, 257K)
Appendix D.
Baseline Assessment: Indications for the Primary Outcome (PDF, 737K)
Appendix E.
Health Care Outcome Survey for Caregivers (PDF, 182K)
Appendix F.
Final Survey for Caregivers (PDF, 180K)
Appendix G.
PCP Survey (PDF, 151K)
Appendix H.
Supplementary Materials (PDF, 1.5M)
Figure S1. The idealized enrollment table (PDF, 164K)
Figure S4. Caregiver Checklist snapshot (PDF, 187K)
Figure S5. Primary care provider (PCP) plan (PDF, 224K)
Figure S6. Study procedure for two-arm randomized control trial of DSC2U (PDF, 147K)
Figure S7. Survey implementation at various time points (PDF, 129K)
Figure S6. Sample size requirements for a range of effect sizes and drop-out rates (PDF, 120K)
Table S1. Baseline characteristics of people with Down syndrome and caregivers (additional variables) (PDF, 183K)
Table S2. Characteristics of PCPs (additional variables) (PDF, 203K)
Supplemental Table S3c. Completion of indicated evaluations (missing evaluations excluded) (PDF, 165K)
Suggested citation:
Chung J, Donelan K, Macklin EA, et al. (2020). Does a Web-Based Platform for Caregivers Help People with Down Syndrome Get Recommended Health Services? Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/10.2020.AD.150731567
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- A randomized controlled trial of an online health tool about Down syndrome.[Genet Med. 2021]A randomized controlled trial of an online health tool about Down syndrome.Chung J, Donelan K, Macklin EA, Schwartz A, Elsharkawi I, Torres A, Hsieh YG, Parker H, Lorenz S, Patsiogiannis V, et al. Genet Med. 2021 Jan; 23(1):163-173. Epub 2020 Sep 3.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers.[Cochrane Database Syst Rev. 2020]The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers.Bajwah S, Oluyase AO, Yi D, Gao W, Evans CJ, Grande G, Todd C, Costantini M, Murtagh FE, Higginson IJ. Cochrane Database Syst Rev. 2020 Sep 30; 9(9):CD012780. Epub 2020 Sep 30.
- Review Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses[ 2014]Review Evidence Brief: The Quality of Care Provided by Advanced Practice NursesMcCleery E, Christensen V, Peterson K, Humphrey L, Helfand M. 2014 Sep
- Review Do Video House Calls With a Specialist Help Get Care to People With Parkinson's Disease?[ 2018]Review Do Video House Calls With a Specialist Help Get Care to People With Parkinson's Disease?Dorsey ER, Achey MA, Korn RE, Arky A, Beck CA, Biglan KM, Stevenson EA, Zhu W, Mammen JR, Elson MJ. 2018 Aug
- Does a Web-Based Platform for Caregivers Help People with Down Syndrome Get Reco...Does a Web-Based Platform for Caregivers Help People with Down Syndrome Get Recommended Health Services?
Your browsing activity is empty.
Activity recording is turned off.
See more...
