This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Human papillomavirus (HPV) causes a number of cancers and other diseases, several of which disproportionally affect Latinos. Unfortunately, Latinos also have poor uptake of HPV vaccines, which prevent HPV-associated diseases. There is a paucity of research on interventions to increase HPV vaccination among Latinos, which our work sought to address.
Objectives:
The objectives of the project were to (1) use community input to create a web-based educational intervention for Latino young adults (aged 18-26) and parents of Latino adolescents (aged 9-17) that provided individually and culturally tailored information about HPV for Latinos; (2) compare the impact of the intervention vs an untailored web-based educational intervention based on the HPV Vaccine Information Sheet from the Centers for Disease Control and Prevention, vs usual care (individualized physician discussion of the vaccine and/or receipt of a paper version of the on HPV Vaccine Information Sheet) on vaccine use among young adults and adolescents.
Methods:
In phase I, community input representing end-users of our intervention created the intervention for Latinos. In phase II, we evaluated this new intervention, called CHICOS (Combatting HPV Infection and Cancers), in a 3-armed, randomized controlled trial conducted in the waiting rooms of 5 family medicine practices serving mainly Latinos. We assessed the relative efficacy of CHICOS vs an untailored intervention, both of which were delivered via iPad, vs usual care for their impact on HPV vaccine uptake among adolescent and young adult Latinos. Eligible participants were English- or Spanish-speaking young adult patients (aged 18-26 years) or parents of Latino adolescents (aged 9-17 years) who reported not yet completing the 3-dose HPV vaccine series (ie, 0, 1, or 2 doses). Data on the primary outcomes—6 different measures of HPV vaccine uptake—came from the clinics' medical records and the statewide immunization registry. The primary analyses were 2-way comparisons between arms (ie, tailored vs untailored, tailored vs control, untailored vs control), stratified by adolescent vs young adult vaccination. We used multiple imputation to account for missing data and analyzed data using an intent-to-treat (ITT) approach. Secondary outcomes included changes in vaccination intention from before to after viewing the iPad materials (tailored and untailored arms only), time between study enrollment and HPV vaccine doses received (all arms), and patterns of utilization of the tailored and untailored websites.
Results:
Significant Latino community input through a series of 6 focus group and quarterly meetings with a community advisory board in phase I was used to create CHICOS. Phase II enrolled 1294 parents and young adults. In the ITT analyses, nearly all vaccination outcomes assessed in 2-way comparisons demonstrated no statistically significant differences between groups. The 1 exception was completion of the series among adolescents who entered the study with at least 1 dose. In this analysis, the tailored group performed significantly better than the untailored group (odds ratio [OR], 2.0; 95% CI, 1.1-3.8) but there were no statistically significant differences in completing the 3-dose series among this subgroup when the tailored intervention was compared against usual care (OR, 1.6; 95% CI, 0.8-3.2). Few young adults received HPV doses. Individuals in the untailored arm took a statistically nonsignificant longer time to receive vaccine doses than those in the untailored or usual care arms. The untailored and tailored interventions significantly improved vaccination intention from baseline to postintervention; the amount of change was the same in the 2 groups.
Conclusions:
Neither the tailored intervention nor the untailored intervention improved HPV vaccination outcomes in adolescents and young adults. In young adults, the rate of HPV vaccination was uniformly low across the 3 study arms, resulting in high rates of missing data.
Background
This application discusses the development and assessment of an educational website called CHICOS (Combatting HPV Infection and Cancers), which the investigators created with an overall goal to inform parents of Latino adolescents and young adult Latinos about human papillomavirus (HPV) infection and vaccination in a culturally relevant and sensitive way.1 The overarching goal of the project was to understand whether this web-based intervention designed to address the unique attitudinal, infrastructural, and insurance/cost barriers faced by individual Latino parents and young adults in making decisions about the HPV vaccine could increase HPV vaccine utilization.
HPV is a family of viruses transmitted mainly through intimate sexual contact. Consistent with the US population overall, Latinos have a high prevalence of HPV infection, estimated to affect more than 80% of sexually active adults during their lifetime.2 Most infections (~75%) occur during adolescence and young adulthood.3,4 Diseases caused by HPV include cervical, oropharyngeal, anal, vaginal, penile, and vulvar cancers, and noncancerous conditions such as cervical precancers (ie, abnormal Pap smears) and genital warts. HPV affects large numbers of both women and men.3-5
More than 14 million people are newly infected with HPV in the United States each year, with 79 million (~12 million Latinos) infected at any given time, exacting a significant emotional, financial, and medical toll.2,5-7 Though HPV infection is similarly prevalent in nearly all race and ethnicity groups, significant disparities in HPV-associated cancers exist for Latinos. For example, Latinas have the highest risk of contracting cervical cancer compared with all other population groups in the US, and Latinos have the highest risk of developing penile cancer.5 Other HPV-related illnesses like genital warts, abnormal Pap smears, anal cancer, and oropharyngeal cancer are also highly prevalent in this population.5
HPV vaccines that protect against the most clinically prevalent types of HPV have been available since 2006 for females and 2009 for males.8 These vaccines are highly effective and, based per-protocol analyses from randomized controlled trials of the vaccine's ability to prevent HPV infection, genital warts, and cervical precancers, their potential to protect against infection with the HPV types responsible for 90% of genital warts cases and 50% of abnormal Pap smears.8,9 At the time we implemented the study, 3 doses of vaccine were required for full protection against HPV infection, and were preferentially recommended for adolescents so as to provide the vaccine before exposure to the virus, which typically occurs shortly after the onset of becoming sexually active. In 2015, after the study was complete, the recommendation changed to 2 doses of vaccine if the series was initiated before age 15, and 3 doses of vaccine if the series was initiated at age 15 or older. Although the vaccine has been recommended by the Centers for Disease Control and Prevention (CDC) for the past several years for routine use among all adolescents aged 11 years and older, vaccination rates have been, and continue to remain, low in the United States.10 As of 2015, it was estimated that only 41.9% of girls and 28.1% of boys aged 13 to 17 nationally had received the full 3-dose vaccine series.11 Vaccination rates are slightly higher among Latinos (46.2% for girls, 35.0% for boys), but still far below that of other adolescent vaccines, and significantly below the national vaccination target level of 80% coverage.12,13 Without significant increases in HPV vaccination, especially among high-risk populations like Latinos, disparities in HPV-related cancers are likely to continue.
Numerous barriers to HPV vaccination have been described in the literature,14-20 including barriers specific to the Latino population.20,21,22-26 These include issues related to parents' concerns about the safety and necessity of HPV vaccines, that children will misconstrue their parents' consent to vaccination as permission to engage in sexual behavior at a young age, and about the vaccine's cost. While understanding the various barriers to HPV vaccination is useful, numerous medical organizations have recently put forth a “call to action” for research on effective interventions to improve HPV vaccine utilization.27-33 However, despite significant effort in this area, few interventions have been shown to have demonstrated effectiveness in improving vaccination rates, especially interventions targeting parents.34 This is a critical gap in knowledge because adolescents are the preferred target population for vaccination, and parents are the “gatekeepers” for adolescent vaccines—parental consent is required for any vaccine to be administered to adolescents younger than age 18. Thus, while the reasons individuals in general, and the Latino population specifically, do not get the HPV vaccine have been well described in the literature, there is a paucity of information on effective interventions to mitigate these issues.
We sought to address this knowledge gap by developing a web-based intervention for Latinos about HPV vaccination. This intervention was driven by community input regarding what information this population wanted and needed when considering the HPV vaccination decision. The resultant CHICOS intervention was novel in that it used individual-level responses to a baseline questionnaire to create personalized educational web pages on HPV infection and vaccination that were responsive to each user's unique informational needs. We then conducted a randomized controlled trial to evaluate the relative efficacy of CHICOS vs an untailored intervention vs usual care in improving HPV vaccination rates among Latino adolescents and young adults. The notion behind this research was that if an intervention could be identified that effectively improved HPV vaccination rates among Latinos, and was widely disseminated, this could lead to eventual decreases in disparities in HPV-related cancers experienced by this population.
In the following Methods and Results sections, information is divided into separate sections to delineate activities involved in phase I (intervention development) and phase II (intervention assessment). A discussion at the end synthesizes results from both phases and contextualizes them within the published literature.
Engagement
While the original idea for the project arose from the research team, stakeholders were involved throughout the project. At the time of the project's conception, the idea for the study (that is, creating an individualized, web-based educational tool about HPV vaccination for Latinos) was vetted among 3 potential end-users of the intervention (2 parents of adolescents and 1 young adult) at the clinical sites (primary care clinics) where the study was proposed to take place. We provided quotes from these participants as well as letters of support with the original grant proposal to the Patient-Centered Outcomes Research Institute (PCORI), which funded this work. Decisions about stakeholder involvement were made prior to the project starting and are described in more detail in the Methods section below.
After the study began, patient engagement continued throughout the project in the form of an ongoing community advisory board (CAB; described in detail below) throughout the 3-year duration of the project. The CAB, which met quarterly, provided significant input into the intervention design, helped with participant recruitment, facilitated study implementation at the clinics, assisted in data interpretation, helped present results at a national conference, and collaborated on developing a dissemination plan. We continue to be in touch with members of the CAB as we discuss additional plans for the intervention in the future.
Methods
Rationale for the Intervention
The main intervention developed and tested in this study was an educational website, called CHICOS, that provided individually customized information about HPV infection and vaccination to parents and young adults. CHICOS was developed in close collaboration with the CAB, and further informed by focus groups. The scientific premise of the intervention is rooted in the concept of message tailoring. Message tailoring refers to customization of information to each individual and has been shown in a number of web- and paper-based interventions to improve compliance with recommended health procedures across diverse settings, clinical issues, and populations.35,36 It is believed to work by making the information provided more personally relevant, and therefore more engaging and likely to be acted on. Prior studies have demonstrated that there is a wide array of attitudinal barriers to HPV vaccination, that barriers differ depending on whether a patient is considering initiating the series or completing it, that some barriers are culturally specific, and that providers often lack enough time during clinical visits to discuss the HPV vaccine in depth with patients. Thus, our hypothesis was that a message tailoring intervention for HPV vaccination, delivered immediately prior to the clinical visit, could provide parents and young adults with the right kind of personally tailored information that would answer questions or alleviate concerns they may have had about the vaccine. In turn, this would result in increases in HPV vaccine utilization compared with an untailored or usual care intervention.
Phase I: Intervention Development Using Community Feedback
Overview
The first phase of the study used an iterative, community-oriented modification approach to adapt for the Latino population a previous version of a web-based educational vaccine intervention that provided individually tailored messages about HPV and 3 other adolescent vaccines. Described below are the CAB meetings and community focus groups that informed the modifications to this intervention. All study activities were approved by the Institutional Review Board of the University of Colorado Denver. A detailed description of the focus group study, providing more details on the study procedures and results, has been published.1
Study Design and Setting
To develop CHICOS, we modified Teen VaxScene, a website that was developed primarily for a Caucasian population in the Midwest (https://www.teen-vaxscene-info.org/sign-up/). Our modification approach incorporated feedback from a CAB that represented our target population (parents of Latino adolescents and young adult Latinos), and data from a series of community focus groups with members of the target population. CAB meetings were held in a community center meeting room at a location central to the participants (ie, library, rec center). Focus group meetings were held in a community meeting building in Aurora, Colorado.
Participants—CAB Population
Our CAB was derived using a purposeful snowball recruitment strategy and comprised 5 Latino parents of adolescents and 4 young adult Latinas. Though attempted, we were unsuccessful in recruiting male participants for the CAB. We recruited participants from community organizations that serve Latino families, staff from the clinics where the planned randomized trial was to take place, and the general community. Three additional members on the CAB represented community and academic stakeholder agencies serving Latinos.
Participants—Focus Group Population
We recruited 20 Latina young adults and 27 Latino parents of adolescents for focus groups from 5 primary care clinics in Colorado that serve low-income, primarily Latino, medically underserved and migrant farmworker populations as the priority clientele, and from the Denver metro area Latino community. These 5 sites were also the clinics where the randomized controlled trial was to take place. Recruitment occurred via advertisements posted in the 5 clinics and community venues. Eligible parents self-identified as being Hispanic or Latino, were ≥ 18 years old, were able to converse in English or Spanish, and reported having an adolescent between the ages of 11 and 17 years old who had not been fully vaccinated. Eligible young adults self-identified as being Hispanic or Latino, were between 18 and 26 years old, were able to converse in English or Spanish, and had not received all 3 doses of the HPV vaccine (ie, 0, 1, or 2 doses). We considered young adult and parent participants ineligible if they could not converse in English or Spanish, or if they/their adolescent had already completed the 3-dose HPV vaccine series. In the end, 20 Latina young adults and 27 Latino parents of adolescents participated in the focus groups.
Outcomes
Outcomes consisted of qualitative feedback from focus groups and the CAB on ways to modify the Teen VaxScene intervention.
Setting
CAB meetings and focus groups took place in community settings (ie, library, community center) near the participants' homes.
Time Frame
All focus group and CAB meetings related to intervention development occurred over a 12-month period from June 2012 to May 2013.
Research Process, Data Collection, and Data Sources
Our initial CAB meetings focused on ways to potentially modify Teen VaxScene for the Latino population. We solicited feedback regarding the design, navigation, and content of the website. The research team collated these suggestions and used them to develop a focus group guide and related materials for subsequent focus group sessions with English- and Spanish-speaking young adults and parents of adolescents. The focus group guide (English version) is provided in the Appendix.
We then held focus group meetings to solicit broader input about modifying Teen VaxScene content and design for the Latino population, and to further understand the perspectives of this population about factors that impact decisions around initiating and completing the recommended HPV vaccine series. Trained members of the research team conducted 6 focus group meetings, each of which lasted approximately 90 minutes. Three meetings were conducted in English, and native Spanish speakers conducted 3 meetings in Spanish. Facilitators followed a written interview guide that included a discussion about attitudinal, infrastructural, and cultural factors that impact decisions about HPV vaccination. Following this, the groups provided structured feedback on the HPV component of Teen VaxScene, specifically its overall appeal, design, layout, functionality, and content. Groups were also asked to suggest additional information and features to include in the future iteration of the intervention. All focus groups were audio-recorded and transcribed verbatim. Spanish transcripts were translated into English by a native Spanish speaker, and then validated by a second native Spanish speaker for accuracy and clarity.
After the focus groups were complete, the CAB held 3 subsequent meetings to make iterative revisions to the intervention logo, color scheme, and content that incorporated their evolving opinions about the intervention. In these meetings, the CAB also assisted the research team in interpreting results from the focus groups and operationalizing these results into intervention modifications. Two members of the study team collected CAB feedback through detailed written meeting notes and best practices documents, and then combined feedback to complete a final summary. The study team subsequently shared notes with CAB members to ensure all relevant information was adequately captured and modified as needed. The CAB also participated heavily in phase II of the project, detailed below.
Analytical Approach
CAB members reviewed all meeting notes to ensure clarity and completeness. We conducted focus group data analysis using established qualitative content analysis methods.37,38 Two members of the study team reviewed transcripts and CAB meeting notes to categorize responses into themes pertaining to factors impacting decision making regarding HPV vaccination, and suggestions for modifying the intervention. The 2 study team members developed initial code categories independently and then compared and discussed them with a third member of the study team until they achieved code agreement. Analysts then applied the resulting codes to the transcripts, and subsequently met routinely to check new findings, discuss emergent new codes and themes, and assess the preliminary results of the analysis process. Discrepancies in coding were resolved by mutual agreement between the reviewers. Throughout the analytic process, we used the qualitative data software program ATLAS.ti ([computer program]. Version 7.0. Berlin, Germany: Scientific Software Development GmbH) for data organization and management. Comments from the CAB and focus groups were synthesized and summarized into a list of edits for the intervention development team and subsequently incorporated into the final version of the intervention. Ultimately, the CAB renamed the intervention, which was now HPV-specific, to CHICOS (Combatting HPV Infections and Cancers).
Phase II: Intervention Implementation
Study Design Overview
Phase II of the study was a 3-armed randomized controlled trial comparing different strategies for informing decisions about HPV vaccines: (1) the culturally and individually tailored educational website intervention, CHICOS, as described above; (2) an untailored, web-based intervention modeled on the CDC's Vaccine Information Sheet for HPV (these sheets are required to be given to patients in paper format prior to HPV vaccine administration and therefore represented an electronic version of usual care); and (3) “usual care” (described in detail below). We enrolled 2 groups of participants—Latino young adults aged 18 to 26 years who were making the vaccination decision for themselves, and Latino parents of adolescents aged 9 to 17 years old who were making vaccination decisions for the adolescent child. The main outcomes assessed were measures of HPV vaccination uptake (assessed for young adults and adolescents separately), and self-reported decision quality related to HPV vaccine decision making and utilization (assessed for young adults and parents of the adolescents). The main intervention took place in the clinics' waiting rooms during a single clinic visit. The intervention lasted 16 months. Vaccination assessments occurred after 5 additional months of data collection on vaccination outcomes. We provided to participants an optional follow-up survey 3 months after study enrollment to assess their views on the interventions provided and how these may have impacted their vaccination decision making. The study was registered on ClinicalTrials.gov (NCT02145156). All study activities were approved by the IRB of the University of Colorado Denver.
Study Design
The study was an individual-level, 3-armed randomized trial.
Participants Enrollment Procedures
Enrollment for the study was facilitated by 2 methods: (1) self-referral to an informational table/desk set up by research assistants (RAs) in clinic waiting rooms (passive recruitment); and RAs approaching specific individuals sitting in the waiting room to assess interest (active recruitment). For the active recruitment, each morning the RAs reviewed the clinic's appointment schedule to determine if any patients scheduled for that day might be eligible for participation based on age. The RAs approached these patients (or their parents) directly at the time of check-in for their clinic visit to assess their interest and eligibility. Several CAB members were employees at the clinics where participants were enrolled and assisted the research assistants in identifying participants and facilitating recruitment (ie, finding a quiet space to discuss the study with the participant).
Eligibility
The RAs assessed eligibility using a predetermined set of questions programmed onto an iPad. RAs read the questions aloud, in either English or Spanish (depending on participant preference), and directly entered responses into the iPad.
We considered parents of adolescents eligible to participate in the study if they met all of the following criteria:
- Reported they were the parent or primary guardian of an adolescent between the ages of 9 and 17 years
- Reported that the adolescent had not yet received all 3 doses of the HPV vaccine at the time of study enrollment
- Were able to read and converse in either English or Spanish
- Reported that the adolescent was a patient at a participating clinic
We considered young adults eligible to participate in the study if they met the following criteria:
- Were between the ages of 18 and 26 years
- Reported that they had not yet received all 3 doses of the HPV vaccine at the time of study enrollment
- Were able to read and converse in either English or Spanish
- Reported they were a patient at a participating clinic
We excluded potential participants if their/their adolescent's reported age range fell outside that acceptable for HPV vaccination, they were not able to read in English or Spanish, they reported they/their adolescent had already completed the 3-dose HPV vaccine series, or they were not/their adolescent was not a patient at the clinic. So as to gather preliminary data on how the intervention may perform in other groups, Latino ethnicity was not an eligibility requirement, but ethnicity data were collected for participants enrolled in the study.
Consent
The iPad was programmed to recognize eligible participants based on their responses to the eligibility questions. Those who were deemed not eligible were thanked for their interest and told they could not participate. RAs asked those who were eligible about their willingness to participate in the study. If they were willing to participate, the RA obtained written informed consent at that time. Participants were provided with a copy of the consent form that described the study purpose, population, risks and benefits, and voluntary nature of the study, and provided contact information for further questions. Receipt of consent was then input into the iPad to continue the study for that participant.
Enrollment
More than 4600 participants were approached for participation in the randomized controlled trial. Of these, 1585 were not screened for eligibility due to their already participating in the study or lack of interest in the study. Of the 3100 participants screened for eligibility, 1780 were found to be ineligible based on the iPad assessment of answers to eligibility questions, 3 did not consent, and 13 left before being randomized. This left a sample of 1294 participants to be randomized (Figure 1). This cohort comprised the intent-to-treat population for the analysis.
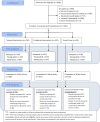
Figure 1
CHICOS CONSORT Diagram for Vaccination Outcomes.
Per-Protocol Population
Shortly after the intervention began, we discovered that we had not instituted a process for collecting adolescents' names from parent participants—a data element needed for matching them to vaccination records. This information was collected for the tailored and untailored groups as part of the baseline survey, but a similar process was not initially in place for the usual care arm as this survey was delivered on paper. This resulted in more than 150 parents in the usual care arm completing the postintervention survey for which we did not have the name of their adolescent and thus could not link to their vaccination records. When we discovered this, the research team reached out by phone and mail to gather this information. These efforts were only partially successful. In the end, we did not know the names of 72 adolescents of parents in the usual care arm. These participants with missing data were eliminated from the per-protocol (PP) analysis, which is presented in the Appendix to this report.
As described above, 1294 participants were randomized. Of these, we could not match 281 to any vaccination or EMR records at the end of the study period and thus also excluded them from both the intention-to-treat and per-protocol vaccination analysis (tailored n = 84, untailored n = 79, usual care n = 118). The main reasons for not being able to match records were variations in the names provided by the parent or participant and the EMR/ Colorado Immunization Information System (CIIS), records which precluded us from being able to definitively conclude there was a match. In addition, some patients indicated they were a patient in the clinic (a requirement for study participation) when in fact they were not—these patients did not have EMR records, and if they had also not been vaccinated, would not have any CIIS records. Of the participants enrolled in the study, 85% had EMR data available, 76% had CIIS data available (not mutually exclusive categories), and 22% participants had no data available in either CIIS or EMR records.
In the per-protocol sample we further eliminated 44 participants who did not complete all elements of the main study and were considered lost to follow-up, 153 participants who were found/whose adolescent was found at the time of vaccination assessment to have already completed the 3-dose HPV vaccination series at the time of study enrollment, and 11 additional participants who were found at the time of vaccination assessment to have been outside the age range for HPV vaccination at the time of study enrollment. With these exclusions, we assessed 805 individuals (tailored n = 280, untailored n = 259, usual care n = 266) in the per-protocol analysis for vaccination status.
Description of Study Interventions and Comparators
Tailored Intervention (CHICOS)
Those in the tailored intervention received an iPad with the CHICOS intervention programmed onto it. This intervention was developed by an academic health communication group that specializes in designing tailored messaging interventions (chcr.umich.edu) and was written at a sixth-grade reading level. The intervention was developed collaboratively with the CAB, which provided feedback on the intervention. The research team conveyed the iterative feedback provided by the CAB to the academic health communication group, which then implemented the changes for further CAB review. Three cycles of review of the intervention were completed in this way to result in the final intervention. A description of this process has been published.1 The intervention commenced with a short baseline survey that collected information about the participants'/participants' adolescent's name and birthday (to link to vaccination records), attitudes and beliefs about HPV infection and vaccination, patient demographics, and vaccination status. This baseline survey was used to individually customize the tailored information in CHICOS that was provided directly on the iPad immediately following completion of the survey. For example, the information was tailored to provide pictures that reflected each participant's self-reported race and gender. Participants entered their/their adolescent's name, which was incorporated throughout. Finally, the content changed depending on the participants' stated concerns. For example, if a participant was most concerned about vaccine safety, information on this topic was provided first on the web page, whereas if a participant was most concerned about efficacy, information on this topic was provided first. Participants viewed the CHICOS information at their own pace for as long as they wished. Following this, they completed a postintervention survey directly on the iPad. The Appendix provides screen shots of the baseline and postintervention surveys and the entire CHICOS intervention.
Untailored intervention
Those randomized to the untailored intervention also initiated the study with the same iPad-based baseline survey as in the tailored intervention. However, information from the survey was not used to customize the information provided by the intervention. Instead, upon completion of the baseline survey the participant was provided with information from the CDC's HPV Vaccine Information Sheet, which had been transcribed verbatim to be shown over a series of 7 web pages. Again, participants could view the information at their own pace for as long as they wished. Following this, they completed a postintervention survey directly on the iPad. The Appendix provides screen shots of the untailored intervention.
Usual care
Those in the usual care arm received whatever care the provider gave during the clinical visit. This varied by provider but based on our prestudy informational interviews with the practices involved in the study typically consisted of bringing up the need for the HPV vaccine during routine physicals (ie, not illness visits) and providing a written version of the Vaccine Information Sheet for HPV at the time the vaccine was administered. However, these activities were completely at provider discretion. Past studies have shown a high degree of variability among providers regarding with which patients HPV vaccines are discussed, under what circumstances, and whether and how the Vaccine Information Sheets are used during visits.39 None of the providers in the usual care arm had access to web-based patient information for HPV. Information on specific HPV-related activities that occurred during the visit was not collected. The patients in the usual care arm did not interact with the iPad directly. They did, however, receive a paper version of the postintervention survey that included the same questions as the iPad-based version of the survey given to those in the tailored and untailored study arms.
Primary Outcome Measures
The primary outcome of interest was HPV vaccine utilization among young adults and adolescent children of the parent participants in the study. We assessed this outcome 5 months after the 16-month study was completed, which meant that individuals had between 5 and 21 months of follow-up time in which to get vaccinated following study enrollment. Vaccination data for adolescents and young adults were planned a priori to be assessed separately. We assessed 6 measures of vaccine uptake in each group:
- Receipt of any needed dose of HPV vaccine during the study period
- Initiation of the series among those who entered the study with 0 doses
- Initiation, but not completion, of the series during the study period among those who entered the study with 0 doses
- Completion of the vaccine series among anyone in the study
- Completion of the series specifically among those entering the study with 1 or 2 doses
- Completion of the series specifically among those entering the study with 0 doses
We included appropriate minimal time intervals between vaccination doses when counting valid doses (ie, doses were not counted as valid if they were erroneously administered too soon after the preceding dose; this occurred in <2% of doses provided). Denominators for the 6 vaccination outcome measures varied based on receipt of prior valid doses and remaining time left in the study period. That is, we eliminated participants from analyses if they did not have enough time left in the vaccination data assessment period to receive the next dose in the series (eg, did not have the minimal 4-month window left in the study period to receive the third dose after a second dose had been administered).
Secondary Outcomes
We assessed 3 secondary outcomes.
Quality of HPV vaccine decision-making
We assessed this outcome for all 3 study arms using questions modified from a validated decision-making scale measure40 that was provided after the visit/intervention in the postintervention survey, and also in the follow-up survey provided by email or postal mail 1 to 2 months after study enrollment. This measure included 4 yes/no questions that we assessed individually.
Patterns of website utilization
We assessed this outcome specifically for the untailored and tailored participants. We used data automatically collected on the iPad server (called paradata) that provide information on the user's navigation through the educational pages.
Timing of vaccine doses
We assessed this outcome in all 3 arms by calculating the time between study enrollment and receipt of any subsequent HPV vaccination doses.
Study Setting
The study took place in the waiting rooms of the 5 primary care clinics enrolled in the trial, which were all part of a single health system. These included 5 family health care clinics in suburban and rural Colorado that serve low-income, primarily Latino, medically underserved clientele.
Time Frame
We recruited parent and young adult participants between June 2014 and September 2015 (with a vaccination follow-up period extending to February 2016).
Data Collection and Sources Randomization
After the RA registered on the iPad that participant consent had been obtained, the iPad used an internal randomization program to assign participants to 1 of the 3 study arms in a 1:1:1 ratio (Figure 1). Because of the possibility of clustering of patient responses to the intervention by their preferred language (Spanish or English), clinic (5 possible sites), type of participant (parent or young adult), and prior HPV doses (none or some), we used these variables as stratification variables in the randomization process to ensure even distribution across study arms. RAs were aware of which study arm participants were assigned to, but the participants and the providers at the clinic were blinded to group allocation.
In-Clinic Participant Flow—Tailored and Untailored Arms
After the eligibility assessment, consent, and randomization, participants in the tailored and untailored arms were given the iPad by the RA and asked to start the intervention. As described above, for the tailored and untailored arms the intervention began with the baseline survey.
Although the participants were encouraged to navigate through the baseline survey on their own, RAs were available to assist participants with questions. Following completion of the baseline survey, the iPad automatically brought participants to either the tailored or untailored website. For both the tailored and untailored arms, when finished viewing the information, participants were prompted by the iPad (and also the RA) to complete a postintervention survey on the iPad directly.
In-Clinic Participant Flow—Usual Care Arm
Those in the usual care arm did not complete a baseline survey or interact with the iPad. Instead, after consent, they continued to their scheduled appointment and received whatever HPV-related information the clinician provided. Data on what occurred during the visit were not collected. After completing their clinic visit, participants returned to the waiting room, where they completed a paper version of the postintervention survey.
Participant Incentives
Each eligible participant who consented to the study and completed the baseline (tailored and untailored arms only) and postintervention surveys (all arms) received a $10 incentive, as they were considered to have “completed” the study.
After-Clinic Follow-up Survey
We assessed parents' and young adult patients' satisfaction with the HPV vaccine decision-making process in an optional follow-up survey. After completion of the in-clinic study procedures, RAs asked participants if they would be willing to fill out a mailed survey 1 to 2 months after their visit. Willing participants provided contact information to the RA at that time. One to 2 months after the enrollment visit, those who chose to participate in this follow-up survey were sent it via postal mail or email. We attempted up to 3 mailings/emailings to solicit a response. Upon completion of the survey, participants were provided an additional $10 incentive. The follow-up survey questions are detailed in the Appendix.
Data Sources
Eligibility data were collected directly into files on the iPad. For the untailored and tailored arms, baseline and postintervention survey data were also recorded on the iPad directly by the participant. All iPad-based information was stored on the website's remote secure server. Data from the postintervention survey for participants in the usual care arm were collected on paper forms and later entered into a REDCap database. We used a similar approach for paper responses to the mailed follow-up survey. Web-based versions of the follow-up survey were collected when users clicked on a live link in an email invitation to the survey that took them to a REDCap site that collected their survey responses directly online into a REDCap database. Data on participants' use of the tailored or untailored websites were tracked automatically through the paradata function embedded in the website and also stored on the remote server. Vaccination data were derived from the clinic's electronic medical records and the statewide immunization registry, Colorado Immunization Information System. We used these 2 data sources for vaccination assessments, as we have found in past studies that this combined approach gives the most comprehensive and valid data. We linked vaccination data between the 2 sources using patient name, birth date, ethnicity, and address when possible. A vaccination event was counted if it was documented in either source.
Colorado Immunization Information System
CIIS is a voluntary immunization registry covering the state of Colorado that includes data from most (75%) private pediatric and family medicine offices in the state, as well as data from all vaccines provided at public health clinics. Internal CIIS audits indicate that more than 85% of Colorado children have >2 immunizations recorded, but utilization of CIIS for tracking adult vaccination is estimated to be much lower (<40%). Offices that use CIIS are required to have <5% error rate in the registry.
Analytical Approach Sample Size
We based the prestudy sample size on an a priori decision to analyze vaccination data for young adults and adolescents separately. We based this decision on the large body of research demonstrating that the barriers faced by these 2 populations regarding vaccination attitudes, access, and insurance coverage are significantly different. Based on previsit vaccination data from the clinic, sample size calculations suggested that we would need at least 573 parents and 426 young adults to be able to detect a 15% difference in vaccination levels between any 2 study arms.
Imputation of Missing Data
Of the 1294 participants, about 22% were missing outcome data, and about 11.5% were missing gender, age, and/or race/ethnicity. Together with these variables, we used site of recruitment, induction date, and arm assignment to develop multiple imputation models,41 separately for young adults and adolescents. We imputed nominal variables with binary or multinomial logistic regression, and quantitative variables using predictive mean matching. We carried out the imputation with the SAS (SAS/STAT 14.1 User's Guide [computer program]. SAS Institute Inc; 2015) MI procedure using the Fully Conditional Specification method.42 For the adolescent data the number of imputations was set at 30 using the percentage missing as a guide.43 For young adults the percentage-missing method yielded only 12 imputations, so the number was reset to the default of 25. We analyzed the resulting data sets individually with logistic regression and performed a combined analysis with the SAS MIANALYZE procedure. Many of the outcomes were very rare in the young adult group, so we used a Firth's penalized maximum likelihood estimation to guard against potential bias.
Primary outcomes
For assessment of HPV vaccination, we stratified all analyses by patient age (young adult vs adolescent). The main analyses were separate 2-way analyses comparing usual care with either the tailored or untailored interventions, and the tailored compared with untailored intervention. We also performed 3-way analyses in which all groups were simultaneously considered. In keeping with methodology standards, intention-to-treat analyses are presented in the main report. PP results are available for the vaccination outcomes in the Appendix.
Secondary outcomes
To evaluate the impact of the different study arms on vaccination timing, we used Cox proportional hazards models examining the number of days between study enrollment and first HPV vaccine dose during the study. We also assessed the number of days between study enrollment and first HPV vaccine dose (ie, series initiation) among the subgroup of participants entering the study unvaccinated. Analyses accounted for clustering at the clinical site.
The primary analysis of the decision quality outcome used chi-square tests to assess variation in this outcome by study arm. To assess vaccination intention over time, Bhapkar's tests of marginal homogeneity and repeated measures binomial regression were the tests of choice as these measures occurred twice per individual (in the postintervention and follow-up surveys). To assess patterns of website utilization, we used t tests to determine differences in time spent by study arm.
Post Hoc Analyses
We performed assessments of baseline survey data as post hoc analyses. We assessed baseline survey data for tailored and untailored arms only, as the usual care group did not receive the baseline survey. The main outcome assessed from the survey was vaccination intention before vs after viewing the educational materials. Assessment occurred in the baseline and postintervention surveys and used a single item that read, “Now that you have read a bit about HPV infection and vaccination, how likely would you be to get a dose of the HPV vaccine today if the doctor recommended it?” We used a 4-point Likert scale (Very Unlikely, Unlikely, Likely, Very Likely) to measure this outcome. We compared analyses between arms and by age (young adult vs parent of an adolescent). Since this was a post hoc assessment, we did not impute missing data.
Role of the CAB
We presented all results to the CAB members over a series of meetings. We asked the CAB to help the study team interpret the data findings and to provide hypotheses to explain the lack of recruitment of males to the young adult portion of the study and poor vaccination uptake among young adults overall. Following this, the CAB helped the study team identify ways to disseminate the findings and the intervention itself to other members of the Latino population.
Modifications to Original Clinical Trials Record
There were modifications to the ClinicalTrials.gov record due to an error in the initial submission and lack of familiarity with how results would ultimately need to be reported to the registry. Initially when the trial was registered, in May 2014, the primary and secondary outcomes' labels were switched, and the trial period was planned for 18 months. Ultimately the trial lasted only 16 months due to a significant delay in the contracting process with PCORI. When it came time to enter results at ClinicalTrials.gov, the study team became aware that the primary and secondary outcome labels needed to be switched, and also that the format of the vaccination outcomes was not entered at trial registration in a way that would allow the study team to report on all 6 vaccination outcomes. We rectified this in the June 2016 modification to the protocol. It is important to note that no changes occurred to the actual conduct of the trial from that originally planned except for the slight decrease from 18 to 16 months in the study's duration.
Results
Phase I: Intervention Development
Participant Characteristics
Table 1 shows the characteristics of the focus group participants. As depicted, both Spanish and English speakers were well represented, and most parent participants were mothers. Only females attended the young adult focus groups. The CAB consisted of 12 members, including the principal investigator for the project, 2 research team members, 5 parents of Latino adolescents, and 4 young adult Latinas. The CAB also included 2 representatives of community organizations that served Latinos, 1 of whom was also the mother of an adolescent.
Table 1
Demographic Characteristics of Focus Group Participants.
CAB Feedback
CAB members provided discussion-based feedback about the content, look, and feel of the Teen VaxScene website, and about potential ways to modify Teen VaxScene. Based on this feedback, we made modifications prior to the start of the intervention to include basic information about HPV for both genders. Members also helped select the color scheme, design the logo, and review and make iterative changes so the intervention design would appear less clinical, use a more approachable font, incorporate more vibrant colors, and use a more eye-catching logo. Members also suggested that participants could choose the content and photos they wanted to view in more detail as opposed to being presented with all information at once. Finally, members helped devise the name—CHICOS (Combatting HPV Infection and Cancers)—for the revised intervention, which was specifically chosen to reflect the Latino community and also to be inclusive of both males and females (as opposed to CHICAS, which would be reflective of females only).
Focus Group Feedback
Three main themes emerged from the focus groups: barriers to vaccination, the need for additional information about HPV, and intervention modifications. These are described in detail below. Table 2 depicts quotes representative of these themes.
Table 2
Supportive Quotes for the Barriers to Vaccination and Basic Information Themes.
Based on focus group feedback, we identified several barriers to vaccination that should be incorporated into the modified intervention. Participants generally had low levels of awareness about the vaccine and the need for 3 doses. They also perceived that they lacked enough general information about both HPV infection and the HPV vaccine to make an informed decision about whether to get vaccinated and suggested incorporating this information very early into the interventional website. In addition, there was a lack of awareness that HPV could impact males, and that males could get vaccinated. Participants believed the cost of the vaccine to be prohibitive, suggesting that information to address the cost of the vaccine was critical to include in the new intervention. Most parent participants suggested that talking about the vaccine with their adolescent children would be a good conversation-starter about safe sexual practices, but they believed that many parents would likely fear that teens would see the vaccine as a clearance for sexual promiscuity. Particular to participants in the Spanish-speaking focus groups were concerns of future reproductive consequences associated with the HPV vaccine. Spanish speakers also asserted that preventive medicine (such as vaccinations) was not a strong cultural norm and that their families tended to prefer home remedies. In addition, they indicated that many within their community hold strong religious values related to sexual activity and abstinence and would be especially reluctant to get the HPV vaccine, particularly for their adolescent children.
The theme of desiring additional basic information about HPV was pervasive among all of the focus group discussions. Participants requested that we include more introductory information about HPV on the intervention website, along with additional logistical information about the HPV vaccine. Specifically, both parents and young adults indicated that they were interested in learning about how HPV is transmitted, risk of contracting HPV, other health or disease risks related to contracting HPV, what symptoms HPV produces, possibilities for treatment of HPV, and the extent to which males are at risk for HPV. Participants also agreed that they wanted to learn more about research efforts and results pertaining to the HPV vaccine, the optimal age range for getting the vaccine, when to initiate the vaccine series, and when to pursue subsequent doses. Parents in particular expressed the desire to review evidence about whether the vaccine is effective, the vaccine's side effects, and how the vaccine works with the immune system. Parent participants in groups conducted in Spanish requested more information about HPV detection and prevention, as well as resources related to attenuating the cost of the HPV vaccine. For many of these information needs, participants requested access to statistical information (ie, cancer prevalence) that was specific to the Latino population. Table 2 shows supporting quotes for these themes.
Participants' evaluation of Teen VaxScene resulted in 3 subcategories of intervention modifications that would make an HPV-specific educational intervention more useful for Latinos. The first related to streamlining content. Both parents and young adults suggested that the amount of text should be minimized, and that more photos of HPV-related cancers and other diseases should be included. Spanish-speaking parents reiterated the suggestion of our CAB that the photos be optional for viewing and that they contain a warning statement about the photos' graphic nature. The second subcategory pertained to the intervention's look and feel. Most participants described the existing theme as too clinical and suggested that the design incorporate brighter colors and a more appealing, family-oriented logo. In line with minimizing the amount of text to read, both parents and young adults requested that more of the content be presented as graphic representations. The third subcategory incorporated suggestions about technical aspects of the intervention. Specifically, both parents and young adults thought users should be able to choose which disease-related photos they wanted to view and be more easily able to choose the variety of content in order to select that which they wanted to read about in more detail. Finally, participants suggested increased utility for users by including additional navigational features throughout the intervention, such as arrows and pop-up menus describing how to navigate through the site.
Phase II: Intervention Implementation
Primary Outcomes—Vaccination Results
Study sample and demographic characteristics of participants with vaccination data assessed
Table 3 depicts the demographic characteristics of participants analyzed for vaccination data. Notably, no male young adults enrolled in the study. Most participants in the study were Hispanic. However, most participants selected “other” race. Anecdotal information from the RAs indicated that many participants identified “Hispanic” as their race, but because this choice was not available on the race question, they chose “other” instead.
Table 3
Demographic Characteristics of Participants With Vaccination Data Assessed.
Impact of the Intervention on HPV Vaccination Levels
Table 4 depicts the 3-way comparisons of HPV vaccination levels by study arm among adolescents and young adults. Overall, few young adults in any study arm received HPV vaccine doses, with only 2% to 6% of those eligible receiving a dose over the 16-month study period.
Table 4
HPV Vaccine Uptake in Young Adults and Adolescents Across the 3 Study Arms.
There were no statistically significant differences by study arm in any of the vaccination uptake measures in 3-way comparisons. The 2-way comparisons for both young adults and adolescents showed the same result (Table 5). The one exception was adolescents who entered the study with at least 1 dose. For these adolescents, the tailored group performed significantly better than the untailored group (odds ratio [OR], 2.0; 95% CI, 1.1-3.8), but there were no statistically significant differences in series completion when the tailored intervention was compared with usual care (OR, 1.6; 95% CI, 0.8-3.2).
Table 5
Two-Way Comparisons of Adolescent Vaccination Between Study Arms.
Secondary Outcomes—Vaccination Timing, Decision Quality, and Website Utilization Impact of the Intervention on Vaccination Timing
Table 6 depicts analysis of the timing of HPV vaccine doses by study arm. There was a general pattern of vaccine doses being administered sooner after enrollment in the tailored arm than in either the usual care or untailored arms. However, none of these differences in vaccination timing were statistically significant.
Table 6
Time to Vaccination, by Study Arm.
Impact on HPV Decision Quality
We used 4 items to measure vaccination decision quality after participants had viewed the intervention materials or, in the case of the usual care group, completed their normal provider visit without interacting with the iPad. In the postintervention survey (Table 7), only the item asking “Are you clear about which benefits and risks matter most to you?” demonstrated significant differences between study arms. The usual care group had a lower rate (71%) of “Yes” responses than either the untailored (87%) or tailored (85%) groups (P = .001).
Table 7
HPV Vaccination Decision Quality; Postintervention Survey.
In the follow-up survey (Table 8), the item “Are you clear about which benefits and risks matter most to you?” again demonstrated significant differences between study arms, where the tailored and untailored invention groups had significantly higher rates of “Yes” responses (78%) compared with the usual care group (65%; P = .006). Additionally, we also observed differences by arm for the item “Do you feel like you know enough about the benefits and risks of getting the vaccine versus not getting the vaccine?” In this instance, participants who viewed the tailored intervention had a larger percentage of “Yes” responses (76%) than the untailored (73%) and the usual care arms (60%; P = .0015).
Table 8
HPV Vaccination Decision Quality: Follow-up Survey.
Website Utilization Patterns
We assessed total amount of time spent on the intervention web pages (Table 9). Participants who viewed the tailored intervention materials spent significantly longer (2.64 minutes) compared with those who viewed the untailored materials (0.76 minutes; P < .001). Those who chose Spanish as their preferred language for the intervention spent close to the same amount of time (1.78 minutes) as those who chose English as their preferred language (1.55 minutes; P = .11). Young adults and parents spent the same amount of time viewing intervention pages.
Table 9
Website Utilization Pattern Based on Condition.
Post Hoc Analyses of Baseline Survey Data
Table 10 depicts the demographic characteristics of participants in the untailored and tailored arms who completed the baseline survey (the survey was not provided to the usual care group). There were no substantial differences (P = .91) between the tailored and untailored arms in the proportion of participants who self-reported acceptance of the HPV vaccine—that is, they indicated they were “very likely” to get the vaccine after viewing the content (Table 11). With both study arms combined (Table 12), the proportion of participants who responded they would be “very likely” to get the HPV vaccine increased between the baseline and postintervention surveys for both parents (72%-77%) and young adults (49%-60%; P < .0001; Figure 2). As shown in Table 12, parents of adolescents were generally more accepting of the vaccine than young adults were. However, comparing baseline to postintervention surveys, young adults had a greater absolute percentage point increase in their vaccine acceptance rates than parents (young adults, 11% increase from 49%-60%; parents, 5% increase from 72%-77%; P = .12).
Table 10
Demographic Characteristics of Participants in the Tailored or Untailored Study Arms Who Completed the Baseline and Postintervention Surveys.
Table 11
HPV Vaccination Intentions Before and After Viewing Educational Materials, Tailored vs Untailored Arms.
Table 12
HPV Vaccination Intentions Before and After Viewing Educational Materials, Young Adults vs Parents.
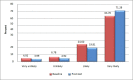
Figure 2
Overall Likelihood to Receive the HPV Vaccine Before vs After Viewing Web-Based Material (P < .0001, Tailored and Untailored Arms Combined).
Discussion
Decisional Context of the Study Results
In this 2-phase project we modified an existing immunization-related, web-based, tailored educational intervention to focus on improving HPV vaccination among the Latino population in the Denver metro area. We then tested the relative efficacy of this intervention, called CHICOS, vs an untailored intervention or usual care for improving HPV vaccination among Latino adolescents and young adults. For the first phase of the study, we successfully engaged a community advisory board consisting of end-users of our product and other community stakeholders focused on the Latino population to create an intervention that incorporated their feedback and that from a series of community focus groups. In the second phase, we found that use of CHICOS resulted in no differences compared with the untailored intervention and usual care in nearly all vaccination outcomes assessed. This was true for both adolescents and young adults, though conclusions about the impact of CHICOS on young adults is somewhat limited by the low vaccine utilization across all arms in this population. Moreover, while vaccination intention improved after reading the educational material in both the tailored and untailored groups, the improvement was the same in the 2 study arms. From this we conclude the CHICOS was not an effective way to improve HPV vaccination among the Latino population targeted for the study.
Study Results in the Context of Prior Research
Increasing HPV vaccination, particularly among adolescents, for whom the vaccine is preferentially recommended, is a national health priority.27-33 Many groups have recently sought to develop and test interventions to improve this outcome, and several effective interventions targeted at the provider or clinic level have been identified. For example, in a 2010 study, Fiks et al revealed that electronic medical record prompts plus parent reminders increased HPV vaccine initiation among females (males were not included in the study) by 9% compared with the control (from 16% to 25%; P < .001).44 Another study by Perkins et al of a provider-focused intervention that provided a combination of education, feedback on vaccination rates, and quality improvement incentives found a 10% to 20% increase (depending on month of analysis) in adolescent HPV vaccine initiation compared with controls.45 Interventions targeted to parents have been less successful as the vast majority of these studies use parental intention for adolescent vaccination as the outcome of interest rather than actual adolescent HPV vaccine receipt.34,46 While several studies have demonstrated improvements in parental vaccination intention with a variety of interventions, the degree to which these improvements translate into actual increases in vaccination remains unclear. This was borne out again in our study—among young adults in the study, vaccination intentions increased significantly over baseline in both the tailored and untailored arms. Despite this, only 2% to 6% of the overall young adult population received any dose of the vaccine during the study period. It is clear from this study that vaccination intention did not translate well into vaccination behaviors, which suggests that researchers should use caution when interpreting results of intervention studies that use vaccination intention as a proxy measure for efficacy regarding vaccine uptake.
Implementation of Study Results
Our study was essentially a negative trial, with no significant differences in vaccine uptake between the study arms. Thus, we do not believe that it is likely that our intervention will be implemented widely in the future, at least in its current state. Should future studies find different results in other populations, for example non-Latinos, then our intervention could be scaled for relatively low cost given that it is web based. However, a major barrier to doing so would be finding ways to prompt participants to engage in the intervention independently. In our study, a research assistant was present throughout the participants' engagement with the intervention and could answer any questions that arose during this interaction (anecdotally, this occurred frequently). To make the intervention sustainable and feasible to implement in a clinic setting more broadly, it would be essential to make sure that participants are capable and willing to engage with the intervention independently.
The low vaccination rate in the young adult population, despite high vaccination intentions, suggests significant and systematic barriers that could not be overcome by the information the interventions provided. The most likely cause of this relates to insurance coverage. Adolescents without health insurance are able to get vaccinated free of charge under the Vaccines For Children program from the US government. This program provides free vaccines to all children of certain ethnicities (eg, American Indians) and/or to those who are underinsured or uninsured for vaccines.47 A similar program does not exist for adults. This means that individuals older than the age of 18 who want the HPV vaccine must pay for it out of pocket. The cost, which averages about $500 for the full 3-dose vaccination series, is prohibitive for many people. This was an issue for the young adult Latino population seen in these clinics, specifically because many of them are undocumented and therefore have no insurance coverage, likely contributing to the low levels of HPV vaccination among young adults across all the study arms. Although our study did not collect insurance information, we believe lack of insurance was a significant driver of the low levels of HPV vaccination seen among young adults. Prior to the study we had confirmed that the clinics had in place a vaccine assistance program run by 1 of the HPV vaccine manufacturers that enables low-income individuals to receive a free vaccine after filling out an online form, regardless of insurance status. This program was available at each of the 5 primary care clinics in the trial, and information on the program was also included in the tailored intervention for young adults. However, feedback from the office staff indicated that the process for enrolling patients in this program was extremely cumbersome, and thus access to the vaccine was a significant barrier for most uninsured patients. Moreover, our CAB suggested that registering for the program may be construed as risky by Latino individuals living in Colorado illegally, which also may have contributed. The extent to which the CHICOS intervention impacts the Latino young adult population, particularly those with health insurance, therefore remains unknown.
Generalizability
Men were not well represented in our study. The entire CAB was composed of females, most of the focus group participants were women, and the young adult population enrolled in our clinical trial was exclusively women, even though gender was not one of the eligibility criteria for enrollment. In contrast, there was nearly equal representation of male and female adolescents among parents enrolling in the study. This low level of adult male participation may reflect young adults' misperceptions that HPV is a “woman's problem” and thus not very relevant to males. Consistent with this misperception are our focus group data, which show a general lack of knowledge about the risk of HPV infection and cancer among males and knowledge that males can get the vaccine, and the finding from our trial that, among adolescents, vaccination was more likely to occur among girls than boys across all study arms.
Lack of male participation in the trial was somewhat disappointing, as we made a concerted effort to highlight in CHICOS the risk of HPV infection and the need for HPV vaccination among people of both genders, as was advised by our CAB. Without this perspective, one cannot make conclusions about the efficacy of our intervention for young adult males. Lack of male participation in young adult focus groups and in the CAB could mean we failed to include important messages in our intervention that would motivate young men to get vaccinated. However, given the lack of impact from CHICOS in vaccination outcomes in other populations represented in the study, we suspect that CHICOS would similarly lack impact for males.
Subpopulation Considerations
The study was underpowered for comparing vaccination rates in subgroups (eg, adolescents vs young adults).
Study Limitations
It is necessary to view this project's results in light of several important limitations. Regarding phase I of the project, the CAB and focus group results were based on people who volunteered to be involved in these discussions. These participants may therefore be more involved in community issues, have higher health literacy, or have stronger opinions about the HPV vaccine than average end-users for the intervention. Second, it is possible that the social desirability bias caused focus group participants to provide more desirable feedback about the website when facing the research team. Mitigating this somewhat is the consistency of our focus group data with that of others regarding barriers to HPV vaccination,48-57 and the fact that we have had an additional 2 years of ongoing feedback from the CAB after these data were collected and integrated into the website without substantial changes in their opinions about the current CHICOS intervention.
Regarding phase II of the project, a significant limitation to the study was that there was a high proportion of missing data, which required imputation. The result of this process was very high uncertainty in the point estimates from the analysis (some 95% CI extended above an OR of 700). In the PP analysis, which was based on the significantly lower number of participants who had full data available, our study ended up being significantly underpowered to detect the differences between arms we had anticipated (15%). The decreased sample size was due primarily to the fact that a substantial proportion of the individuals whose vaccination status we tried to assess could not be matched to any vaccination record in CIIS or any of the clinic's electronic medical records. This primarily impacted adolescents and was usually because the name the parent wrote down as his or her adolescent's name at the time of study enrollment could not be definitively matched to a record, even when birth date was added as a parameter. For an additional 72 parents in the usual care arm, we had a problem with our initial study processes in which we failed to record the adolescent's name, also preventing us from being able to link these parents' responses to adolescent vaccination outcome. Moreover, we eliminated a number of additional participants from the per-protocol analysis because they erroneously reported that they/their adolescent had not completed the 3-dose series at the time of study enrollment when in fact they had completed the series (as judged by their vaccination records, which were reviewed at the end of the study period), and/or that they were in the eligible age range when they were not. It is possible that some participants purposefully misrepresented their/the adolescent's age or vaccination status in order to participate in the study. However, prior research demonstrates that parents (and to some extent young adults) are often unaware of how many doses of the HPV vaccine they have received previously,58 which could have also played a role. In either case, the removal of so many participants from the PP analysis due to lack of eligibility or inability to match to clinical records was not expected and hindered our statistical power to detect differences between study arms. In developing our study, we had hypothesized that the untailored intervention would be better than usual care, but inferior to the tailored intervention CHICOS. Our study was powered to identify a 15% difference between study groups, which was not achieved for any of our outcomes.
Another limitation from phase II of the study is that participants were interested in participating when approached by RAs. Participants may have thus already had fairly positive attitudes toward HPV vaccination, which could account in part for the high baseline levels of HPV vaccination intention reported in both the tailored and untailored arms. Also, many participants requested RA assistance in using the intervention. Because of this, social desirability may have influenced our results, particularly related to vaccination intention, as study subjects might not have felt comfortable expressing hesitancies about the HPV vaccine or ambiguous intentions about getting vaccinated directly to the RA. The need for RA assistance to use the iPads also raises questions about how easy or difficult it would be for participants to use the intervention on their own, an important issue to consider when determining the feasibility of disseminating iPad based widely. Mitigating this concern somewhat are national data showing that Latinos are more likely than any other race or ethnicity group to look for health information on the web, particularly using smart phone technology.59,60 Finally, our CHICOS intervention was clinic based and therefore captured patients with ready access to care (and thus the vaccine). It is not known how effective our intervention would be in high-risk populations that do not routinely access primary care.
Future Research
Though our intervention was not effective at improving vaccination rates among Latino adolescents and young adults, it is possible that the intervention could be effective in other populations. Thus, future research should continue to examine the overall efficacy of tailored messaging for improving HPV vaccination, as HPV vaccination rates are below national goals in essentially all subpopulations.
Conclusions
The CHICOS intervention, which we developed in close collaboration with the Denver metro area Latino community, did not appear to be any more effective than an untailored web-based intervention or usual care for improving HPV vaccination rates among Latino adolescents or young women. While both the tailored and untailored interventions significantly improved parents' and young adults' HPV vaccination intentions, this did not appear to translate into improvements in actual HPV vaccine uptake. This was particularly notable in the young adult population, which had very low levels of HPV vaccine utilization across all 3 study arms, likely reflecting substantial systematic barriers to vaccination that could not be overcome by the information provided. Ongoing research is needed to identify effective mechanisms to improve HPV vaccination among Latinos, a high-risk group for HPV-related cancers and other diseases.
Definitions
- Decision quality related to HPV vaccination
How comfortable participants feel with their decision to get or not get the vaccine, assessed with a variety of validated measures
- Entering the study unvaccinated
Enrolling the study without any prior HPV vaccine doses
- Vaccine acceptance
Generally agreeing with receiving the vaccine, but not necessarily stating it
- Vaccine initiation
Receiving the first of the needed 3 doses in the HPV vaccine series
- Vaccination intention
Specifically stating that one plans to get (or have one's adolescent get) vaccinated
- Vaccine series completion
Receiving all 3 doses in the HPV vaccine series
- Vaccine uptake
Receiving a vaccine dose
Abbreviations
- CAB
community advisory board
- CDC
Centers for Disease Control and Prevention
- CHICOS
Combatting HPV Infection and Cancers, an individually tailored, web-based educational intervention
- CIIS
Colorado Immunization Information System, a statewide vaccine registry
- EMR
Electronic Medical Record
- HPV
human papillomavirus
- PCORI
Patient-Centered Outcomes Research Institute
- PP
per protocol
- RA
research assistant
References
- 1.
- Maertens J, Zambrano-Jimenez AM, Albright K, Dempsey AF. Using community engagement to develop a web-based intervention for Latinas about the HPV vaccine. J Health Commun. 2017;22(4):285-293. [PubMed: 28276945]
- 2.
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187-193. [PubMed: 23403598]
- 3.
- Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194(8):1044-1057. [PubMed: 16991079]
- 4.
- Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813-819. [PubMed: 17327523]
- 5.
- Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175-201. [PMC free article: PMC3565628] [PubMed: 23297039]
- 6.
- Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660-664. [PMC free article: PMC6745688] [PubMed: 25299412]
- 7.
- Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016-6019. [PMC free article: PMC6629018] [PubMed: 22867718]
- 8.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(RR-2):1-24. [PubMed: 17380109]
- 9.
- Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(RR-05):1-30. [PubMed: 25167164]
- 10.
- Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014 - United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620-624. [PMC free article: PMC5779422] [PubMed: 25055185]
- 11.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850-858. [PubMed: 27561081]
- 12.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792. [PMC free article: PMC4584833] [PubMed: 26225476]
- 13.
- Koh HK, Blakey CR, Roper AY. Healthy People 2020: a report card on the health of the nation. JAMA. 2014;311(24):2475-2476. [PubMed: 24870206]
- 14.
- Wong CA, Berkowitz Z, Dorell CG, Anhang Price R, Lee J, Saraiya M. Human papillomavirus vaccine uptake among 9- to 17-year-old girls: National Health Interview Survey, 2008. Cancer. 2011;117(24):5612-5620. [PMC free article: PMC3179804] [PubMed: 21692069]
- 15.
- Constantine NA, Jerman P. Acceptance of human papillomavirus vaccination among Californian parents of daughters: a representative statewide analysis. J Adolesc Health. 2007;40(2):108-115. [PubMed: 17259050]
- 16.
- Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117(5):1486-1493. [PubMed: 16651301]
- 17.
- Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Ann Epidemiol. 2009;19(8):531-538. [PMC free article: PMC2880849] [PubMed: 19394865]
- 18.
- Patel DA, Zochowski M, Peterman S, Dempsey AF, Ernst S, Dalton VK. Human papillomavirus vaccine intent and uptake among female college students. J Am Coll Health. 2012;60(2):151-161. [PMC free article: PMC3307218] [PubMed: 22316412]
- 19.
- Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: findings from the 2006-2008 National Survey of Family Growth. Vaccine. 2012;30(16):2676-2682. [PubMed: 22342548]
- 20.
- Bastani R, Glenn BA, Tsui J, et al. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1463-1472. [PMC free article: PMC3132826] [PubMed: 21602307]
- 21.
- Kobetz E, Kornfeld J, Vanderpool RC, et al. Knowledge of HPV among United States Hispanic women: opportunities and challenges for cancer prevention. J Health Commun. 2010;15 Suppl 3:22-29. [PMC free article: PMC3858859] [PubMed: 21154081]
- 22.
- Casillas A, Singhal R, Tsui J, Glenn BA, Bastani R, Mangione CM. The impact of social communication on perceived HPV vaccine effectiveness in a low-income, minority population. Ethn Dis. 2011;21(4):495-501. [PubMed: 22428357]
- 23.
- Flores K, Bencomo C. Preventing cervical cancer in the Latina population. J Womens Health (Larchmt). 2009;18(12):1935-1943. [PubMed: 20044855]
- 24.
- Bair RM, Mays RM, Sturm LA, Zimet GD. Acceptability of the human papillomavirus vaccine among Latina mothers. J Pediatr Adolesc Gynecol. 2008;21(6):329-334. [PubMed: 19064226]
- 25.
- Lechuga J, Swain GR, Weinhardt LS. The cross-cultural variation of predictors of human papillomavirus vaccination intentions. J Womens Health (Larchmt). 2011;20(2):225-230. [PMC free article: PMC3064873] [PubMed: 21314448]
- 26.
- Lechuga J, Swain GR, Weinhardt LS. Impact of framing on intentions to vaccinate daughters against HPV: a cross-cultural perspective. Ann Behav Med. 2011;42(2):221-226. [PMC free article: PMC5045861] [PubMed: 21533624]
- 27.
- National Vaccine Advisory Committee. The promise and challenge of adolescent immunization. Am J Prev Med. 2008;35(2):152-157. [PubMed: 18617084]
- 28.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 558: Integrating immunizations into practice. Obstet Gynecol. 2013;121(4):897-903. [Article retracted] [PubMed: 23635707]
- 29.
- Zimet GD. Health care professionals and adolescent vaccination. A call for intervention research. Hum Vaccin Immunother. 2014;10(9):2629-2630. [PMC free article: PMC4975052] [PubMed: 25483506]
- 30.
- Center for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, Health Communications Office. You are the key to HPV cancer prevention: communicating about HPV. 2014. Accessed September 30, 2014. https:
//immunizationcoalitions .org/content /uploads/2014/03/hpv-cdc.pdf - 31.
- Vanderpool RC, Crosby RA, Stradtman LR. Protecting a new generation against HPV: are we willing to be bold? Hum Vaccin Immunother. 2014;10(9):2559-2561. [PMC free article: PMC4772861] [PubMed: 25483474]
- 32.
- National Cancer Institute. President's Cancer Panel Annual Report, Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer. A Report to the President of the United States from the President's Cancer Panel. 2014.
- 33.
- American Academy of Pediatrics. Give a strong recommendation for HPV vaccine to increase uptake. 2015. Accessed January 15, 2016. http://www.immunize.org/letter/recommend hpv vaccination.pdf
- 34.
- Dempsey AF, Zimet GD. Interventions to Improve Adolescent Vaccination: What May Work and What Still Needs to Be Tested. Vaccine. 2015;33 Suppl 4:D106-113. [PubMed: 26615169]
- 35.
- Lustria ML, Noar SM, Cortese J, Van Stee SK, Glueckauf RL, Lee J. A meta-analysis of web-delivered tailored health behavior change interventions. J Health Commun. 2013;18(9):1039-1069. [PubMed: 23750972]
- 36.
- Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133(4):673-693. [PubMed: 17592961]
- 37.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15(9):1277-1288. [PubMed: 16204405]
- 38.
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Education Today. 2004;24(2):105-112. [PubMed: 14769454]
- 39.
- Davis TC, Fredrickson DD, Arnold CL, et al. Childhood vaccine risk/benefit communication in private practice office settings: a national survey. Pediatrics. 2001;107(2):e17. [PubMed: 11158491]
- 40.
- O'Connor AM. Validation of a decisional conflict scale. Medical decision making : an international journal of the Society for Medical Decision Making. 1995;15(1):25-30. [PubMed: 7898294]
- 41.
- Fairclough D. Design and Analysis of Quality of Life Studies in Clinical Trials, 2nd Edition. 2010.
- 42.
- SAS/STAT 14.1 User's Guide. SAS Institute Inc [computer program]. 2015.
- 43.
- Von Hippel P. How to impute interactions, squares, and other transformed variables. Socio-logical Methodology 2009;39:265-291.
- 44.
- Fiks AG, Grundmeier RW, Mayne S, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114-1124. [PMC free article: PMC3666111] [PubMed: 23650297]
- 45.
- Perkins RB, Zisblatt L, Legler A, Trucks E, Hanchate A, Gorin SS. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33(9):1223-1229. [PubMed: 25448095]
- 46.
- Zimet GD, Buffler P. Prevention of human papillomavirus-related diseases: Impediments to progress. Prev Med. 2013;57(5):407-408. [PubMed: 23954187]
- 47.
- Obaro SK, Palmer A. Vaccines for children: policies, politics and poverty. Vaccine. 2003;21(13-14):1423-1431. [PubMed: 12615439]
- 48.
- Dempsey AF, Davis MM. Overcoming barriers to adherence to HPV vaccination recommendations. The American journal of managed care. 2006;12(17 Suppl):S484-491. [PubMed: 17203992]
- 49.
- Ferrer HB, Trotter C, Hickman M, Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC Public Health. 2014;14:700. [PMC free article: PMC4100058] [PubMed: 25004868]
- 50.
- Firenze A, Marsala MG, Bonanno V, et al. Facilitators and barriers HPV unvaccinated girls after 5 years of program implementation. Hum Vaccin Immunother. 2015;11(1):240-244. [PMC free article: PMC4514162] [PubMed: 25483543]
- 51.
- Hamlish T, Clarke L, Alexander KA. Barriers to HPV immunization for African American adolescent females. Vaccine. 2012;30(45):6472-6476. [PubMed: 22910288]
- 52.
- Javanbakht M, Stahlman S, Walker S, et al. Provider perceptions of barriers and facilitators of HPV vaccination in a high-risk community. Vaccine. 2012;30(30):4511-4516. [PubMed: 22561142]
- 53.
- Keating KM, Brewer NT, Gottlieb SL, Liddon N, Ludema C, Smith JS. Potential barriers to HPV vaccine provision among medical practices in an area with high rates of cervical cancer. J Adolesc Health. 2008;43(4 Suppl):S61-67. [PubMed: 18809147]
- 54.
- Lechuga J, Vera-Cala L, Martinez-Donate A. HPV Vaccine Awareness, Barriers, Intentions, and Uptake in Latina Women. Journal of immigrant and minority health / Center for Minority Public Health. 2014. [PMC free article: PMC4449312] [PubMed: 25432149]
- 55.
- Levy B. Integrating HPV vaccination into your practice: overcoming common barriers. The Journal of family practice. 2009;58(9 Suppl HPV):S11-13. [PubMed: 19744420]
- 56.
- Pierre Joseph N, Belizaire M, Porter CL, et al. Ethnic Differences in Perceived Benefits and Barriers to HPV Vaccine Acceptance: A Qualitative Analysis of Young African American, Haitian, Caucasian, and Latino Men. Clin Pediatr (Phila). 2014;53(2):177-185. [PubMed: 24403292]
- 57.
- Zimet GD. Potential barriers to HPV immunization: from public health to personal choice. Am J Law Med. 2009;35(2-3):389-399. [PubMed: 19697755]
- 58.
- Attanasio L, McAlpine D. Accuracy of Parental Reports of Children's HPV Vaccine Status: Implications for Estimates of Disparities, 2009-2010. Public health reports (Washington, DC: 1974). 2014;129(3):237-244. [PMC free article: PMC3982544] [PubMed: 24791021]
- 59.
- Pew Internet and American Life Project. Mobile Health 2012. 2012; http://pewinternet.org/Reports/2012/Mobile-Health.aspx, 12/12/12.
- 60.
- Pew Research Center. Latinos and Digital Technology. 2011; http://pewresearch
.org /pubs/1887/latinos-digital-technology-internet-broadband-cell-phone-use. Accessed 09/01/12.
Acknowledgment
Research reported in this report was [partially] funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (1455). Further information available at: https://www.pcori.org/research-results/2012/does-customized-website-affect-hpv-vaccine-use-among-latino-adolescents-and
Appendices
Appendix A.
Parent Focus Group Guide (PDF, 187K)
Appendix B.
Young Adult Focus Group Guide (PDF, 177K)
Appendix C.
Participant Eligibility Survey (PDF, 120K)
Appendix D.
Participant Baseline Survey (PDF, 195K)
Appendix E.
Participant Post Intervention Survey (PDF, 158K)
Appendix F.
Participant Post Intervention Survey – Usual Care Arm (PDF, 185K)
Appendix G.
Participant Follow-up Survey – Parents (PDF, 194K)
Appendix H.
Participant Follow-up Survey – Young Adults (PDF, 193K)
Appendix I.
CHICOS Intervention/Website Screenshots (PDF, 2.5M)
Appendix J.
Basic/VIS Format – Untailored Intervention (PDF, 1.1M)
Appendix K.
Tables 3 to 5 From Report Showing Data From PP Analysis of Vaccination Outcomes (PDF, 262K)
Appendix L.
Exploratory Analysis of Other Factors Impacting Vaccination (PDF, 55K)
Suggested citation:
Dempsey AF, Maertens J, Jimenez-Zambrano A, Sevick C. (2018). Does a Customized Website Affect HPV Vaccine Use among Latino Adolescents and Young Adults? Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/10.2018.CER.1455
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- A randomized, controlled, pragmatic trial of an iPad-based, tailored messaging intervention to increase human papillomavirus vaccination among Latinos.[Hum Vaccin Immunother. 2019]A randomized, controlled, pragmatic trial of an iPad-based, tailored messaging intervention to increase human papillomavirus vaccination among Latinos.Dempsey AF, Maertens J, Sevick C, Jimenez-Zambrano A, Juarez-Colunga E. Hum Vaccin Immunother. 2019; 15(7-8):1577-1584. Epub 2019 Feb 20.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Effect of a Health Care Professional Communication Training Intervention on Adolescent Human Papillomavirus Vaccination: A Cluster Randomized Clinical Trial.[JAMA Pediatr. 2018]Effect of a Health Care Professional Communication Training Intervention on Adolescent Human Papillomavirus Vaccination: A Cluster Randomized Clinical Trial.Dempsey AF, Pyrznawoski J, Lockhart S, Barnard J, Campagna EJ, Garrett K, Fisher A, Dickinson LM, O'Leary ST. JAMA Pediatr. 2018 May 7; 172(5):e180016. Epub 2018 May 7.
- Review Face-to-face interventions for informing or educating parents about early childhood vaccination.[Cochrane Database Syst Rev. 2018]Review Face-to-face interventions for informing or educating parents about early childhood vaccination.Kaufman J, Ryan R, Walsh L, Horey D, Leask J, Robinson P, Hill S. Cochrane Database Syst Rev. 2018 May 8; 5(5):CD010038. Epub 2018 May 8.
- Review The Effectiveness of mHealth Interventions Targeting Parents and Youth in Human Papillomavirus Vaccination: Systematic Review.[JMIR Pediatr Parent. 2023]Review The Effectiveness of mHealth Interventions Targeting Parents and Youth in Human Papillomavirus Vaccination: Systematic Review.Ou L, Chen AC, Amresh A. JMIR Pediatr Parent. 2023 Nov 21; 6:e47334. Epub 2023 Nov 21.
- Does a Customized Website Affect HPV Vaccine Use among Latino Adolescents and Yo...Does a Customized Website Affect HPV Vaccine Use among Latino Adolescents and Young Adults?
Your browsing activity is empty.
Activity recording is turned off.
See more...
