NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ziprasidone is an atypical antipsychotic used in the treatment of adults with schizophrenia and bipolar disorder. Use of ziprasidone has not been consistently associated with serum enzyme elevations but has been linked to rare instances of hypersensitivity reactions accompanied by mild-to-moderate acute liver injury.
Background
Ziprasidone (zi pras' i done) is a benzisothiazolyl piperazine-type atypical antipsychotic that appears to act as both a dopamine type 2 (D2) and a serotonin (5-HT2) receptor antagonist. It also has moderate activity against α-adrenergic and histamine receptors. Ziprasidone is indicated for the therapy of schizophrenia and as either monotherapy or adjunctive therapy for acute manic episodes and maintenance therapy for manic and mixed episodes in bipolar 1 disorder. Ziprasidone was approved for use in the United States in 2001 and is widely used with more than a million prescriptions written yearly in the United States. Ziprasidone is available as capsules of 20, 40, 60 and 80 mg generically and under the brand name Geodon. It is also available as an oral suspension and as a solution for intramuscular injection. The typical initial dose is 20 mg twice daily, which can be increased to a maintenance dose in the range of 40 to 80 mg twice daily. Common side effects include somnolence, dizziness, restlessness, fatigue, headache, nausea, dyspepsia, anorexia, dry mouth and blurred vision. Weight gain is uncommon and extrapyramidal symptoms occur in about 5% of patients. Uncommon but potentially severe adverse events include neuroleptic malignant syndrome, tardive dyskinesia, rash, DRESS syndrome, metabolic changes with weight gain, hyperglycemia and dyslipidemia, orthostatic hypotension, leukopenia, neutropenia, seizures, suicidal ideation and behaviors, increased risk of cerebral vascular incidents and death in the elderly dementia related psychosis.
Hepatotoxicity
Liver test abnormalities have been reported in patients taking ziprasidone, but they have not been well characterized in the literature and the frequency of elevations appears to be similar to placebo therapy. Several instances of hypersensitivity reactions have been reported in patients taking ziprasidone, arising within 1 to 4 weeks of starting therapy and with rapid recurrence on reexposure in at least one case. In several instances, the hypersensitivity reaction qualified as DRESS syndrome with rash, eosinophilia and an accompanying liver injury either in the form of moderate serum enzymes or a mixed hepatitis with jaundice. In all instances, the symptoms, signs and laboratory abnormalities resolved rapidly with stopping ziprasidone. Thus, on rare occasions, ziprasidone can cause acute hypersensitivity reactions that can be accompanied by hepatitis, but the liver injury is usually mild and self-limited.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Ziprasidone is largely excreted unchanged in the urine and its hepatic metabolism is minimal, perhaps accounting for why hepatotoxicity is rare. The liver injury caused by ziprasidone is likely due to hypersensitivity and is idiosyncratic in nature. Ziprasidone is less likely to cause weight gain than other atypical antipsychotics and has not been linked to cases of steatosis.
Outcome and Management
The hypersensitivity reactions to ziprasidone are likely to recur with reexposure and rechallenge should be avoided. Ziprasidone has not been reported to cause acute liver failure, chronic hepatitis or vanishing bile duct syndrome in the published literature. Persons with hypersensitivity to ziprasidone are likely to tolerate other atypical antipsychotic medications, but prospective monitoring is warranted if other such agents are used.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ziprasidone – Generic, Geodon®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
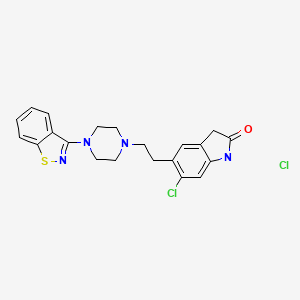
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ziprasidone | 122883-93-6 | C21-H21-Cl-N4-O-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 June 2023
Abbreviations: DRESS, drug reaction with eosinophilia and systemic symptoms.
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20:491–505. [PubMed: 10192829](Controlled trial of ziprasidone in two doses [n=210] vs placebo [n=92] for 6 weeks; “Changes in liver enzymes were notably rare.” Three of 210 patients had ALT above 3 times ULN, but these were judged to be unrelated to drug therapy; further characterization not given).
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. [PubMed: 10553730](Systematic review of 81 articles on weight change with antipsychotics, using change after 10 weeks to compare agents: clozapine +5.7, olanzapine +4.2, chlorpromazine +4.2, risperidone +1.7, loxapine +0.6, haloperidol +0.5, ziprasidone +0.3, molindone -0.1, and pimozide -2.7 kilograms).
- Balestrieri M, Vampini C, Bellantuono C. Efficacy and safety of novel antipsychotics: a critical review. Hum Psychopharmacol. 2000;15:499–512. [PubMed: 12404619](Review on efficacy and safety of antipsychotics; 3 of 702 patients on ziprasidone discontinued therapy because of liver test abnormalities).
- Ziprasidone (Geodon) for schizophrenia. Med Lett Drugs Ther. 2001;43:51–2. [PubMed: 11402259](Brief review of efficacy and safety of ziprasidone shortly after its approval in the US; side effects include somnolence, extrapyramidal symptoms, and transient prolactin elevations; no mention of ALT elevations or hepatotoxicity).
- Arato M, O'Connor R, Meltzer HY., ZEUS Study Group. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17:207–15. [PubMed: 12177583](Controlled trial of placebo vs ziprasidone in 3 doses in 294 patients; laboratory abnormalities reported in similar proportion of ziprasidone as placebo treated subjects, but details of ALT levels not provided).
- Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother. 2002;36:839–51. [PubMed: 11978164](Review of structure, pharmacology, kinetics, metabolism, efficacy and side effects of ziprasidone; no mention of effects on ALT levels or liver injury).
- Choice of an antipsychotic. Med Lett Drugs Ther. 2003;45:102–4. [PubMed: 14679353](Common side effects of ziprasidone are insomnia, anxiety, headache, nausea, constipation and lightheadedness; unlike clozapine, olanzapine, risperidone and quetiapine, it has little or no effect on weight; no mention of hepatic side effects).
- Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28:99–112. [PMC free article: PMC161731] [PubMed: 12670127](Review of the interactions of the atypical antipsychotics with the P450 system; clozapine metabolized by CYP 1A2 and 3A4 and possibly 2C9 and 2D6; risperidone by CYP 2D6 and possibly 3A4; olanzapine by CYP 1A2 and possibly 2D6; quetiapine and ziprasidone by CYP 3A4).
- Tsai CF, Tsai SJ, Hwang JP. Ziprasidone-induced hypersensitivity syndrome in an aged schizophrenia patient. Int J Geriatr Psychiatry. 2005;20:797–9. [PubMed: 16035110](69 year old Chinese woman developed skin rash and fever 11 days after starting ziprasidone followed by jaundice [bilirubin 4.4 mg/dL, ALT 72 U/L, Alk P 398 U/L, eosinophils 5%], with resolution of all abnormalities within 3 weeks of stopping).
- Rettenbacher MA, Baumgartner S, Eder-Ischia U, Edlinger M, Graziadei I, Hofer A, Huber R, et al. Association between antipsychotic-induced elevation of liver enzymes and weight gain: a prospective study. J Clin Psychopharmacol. 2006;26:500–3. [PubMed: 16974192](Prospective study of 67 patients started on atypical antipsychotics [6 on ziprasidone]; ALT elevations were more frequent in 14 patients who gained >7% of body weight than in 53 who did not [50% vs 19%], and mean changes in ALT, AST and GGT were greater in those who gained weight; all abnormalities were transient, asymptomatic and not associated with bilirubin elevations).
- Wright TM, Vandenberg AM. Risperidone- and quetiapine-induced cholestasis. Ann Pharmacother. 2007;41:1518–23. [PubMed: 17666578](30 year old man developed jaundice on risperidone and lithium for 8 years [bilirubin 4.7 mg/dL, ALT 99 U/L, Alk P 267 U/L], resolving with change of risperidone to ziprasidone, but recurrent jaundice 1 year later 3 weeks after starting quetiapine, having tolerated olanzapine).
- Akkaya C, Sarandol A, Aydogan K, Kirli S. Urticaria and angio-oedema due to ziprasidone. J Psychopharmacol. 2007;21:550–2. [PubMed: 17446198](36 year old Turkish woman developed urticaria and facial edema 4 weeks after starting ziprasidone with normal liver tests and no fever, resolving within 3 days of stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008; several antidepressants [duloxetine, sertraline, fluoxetine, amitriptyline], but none of the atypical antipsychotic agents were implicated).
- Torrent C, Amann B, Sanchez-Moreno J, Colom F, Feinares M, Comes M, Rosa AR, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18. [PubMed: 18498432](Review of frequency of weight gain in patients treated for bipolar disorders, most weight gain occurred with clozapine and olanzapine, but some weight gain also with quetiapine, risperidone, lithium, valproate and gabapentin; not with aripiprazole, ziprasidone, carbamazepine or lamotrigine).
- Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–10. [PubMed: 19321312](Analysis of weight gain in 21 placebo controlled trials [~3300 patients]; average monthly weight gain in pounds was +0.1 with placebo, +0.8 olanzapine, +0.6 risperidone, -0.3 ziprasidone. A 5% increase in weight occurred after one year in 13% of placebo, 39% haloperidol, 20% ziprasidone, 45% risperidone and 60% olanzapine treated subjects).
- Sacchetti E, Galluzzo A, Valsecchi P, Romeo F, Gorini B, Warrington L., MOZART Study Group. Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res. 2009;113:112–21. [PubMed: 19606529](Controlled trial of clozapine [300 mg] vs ziprasidone [80-160 mg] daily for 18 weeks; similar efficacy; weight gain +0.8 kg with clozapine vs -2.6 kg with ziprasidone; “no detrimental effects for either drug were observed with regard to liver functions…”).
- Díaz-Marsá M, Sánchez S, Rico-Villademoros F., ZIP-IIG-79 Study Group. Effectiveness and tolerability of oral ziprasidone in psychiatric inpatients with an acute exacerbation of schizophrenia or schizoaffective disorder: a multicenter, prospective, and naturalistic study. J Clin Psychiatry. 2009;70(4):509–17. [PubMed: 19358789](Open label study in 196 inpatients given ziprasidone; no mention of ALT levels or hepatotoxicity).
- Addington DE, Labelle A, Kulkarni J, Johnson G, Loebel A, Mandel FS. A comparison of ziprasidone and risperidone in the long-term treatment of schizophrenia: a 44-week, double-blind, continuation study. Can J Psychiatry. 2009;54:46–54. [PubMed: 19175979](Controlled trial of ziprasidone vs risperidone in 139 patients for 8 weeks; less weight gain with ziprasidone; “One ziprasidone-treated patient had an elevation of liver enzymes and was discontinued from the study”).
- Kim SW, Shin IS, Kim JM, Bae KY, Yang SJ, Yoon JS. Effectiveness of switching from aripiprazole to ziprasidone in patients with schizophrenia. Clin Neuropharmacol. 2010;33:121–5. [PubMed: 20502130](Switching 19 patients with schizophrenia from aripiprazole to ziprasidone resulted in a decline in mean ALT levels [26 to 18 U/L], values becoming normal in 2 of 3 subjects with elevations on aripiprazole).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 4 due to psychotropic agents; one each for quetiapine, nefazodone, fluoxetine and venlafaxine, but none for phenothiazines or ziprasidone).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with suspected drug induced liver injury, half [n=15] were due to antimicrobials [minocycline 4, INH 3, azithromycin 3] and the rest largely due to CNS agents and anticonvulsants; one case was attributed to perphenazine, but none to ziprasidone).
- Gordon JS, Neyman KM, Wells RD, Chen SC. Drug rash with eosinophilia and systemic symptoms (DRESS syndrome). Cutis. 2012;89:180–2. [PubMed: 22611746](27 year old woman developed pharyngitis, facial swelling, rash and fever 2 weeks after starting ziprasidone [bilirubin 2.6 mg/dL, ALT 688 U/L, Alk P 812 U/L, eosinophils 19%], with recurrence 2 days after ziprasidone was restarted [bilirubin 11.5 mg/dL, ALT 2061 U/L], responding to high doses of prednisone and then tolerating risperidone).
- Marwick KF, Taylor M, Walker SW. Antipsychotics and abnormal liver function tests: systematic review. Clin Neuropharmacol. 2012;35:244–53. [PubMed: 22986798](Systematic review of the literature found rates of any serum enzyme elevation during antipsychotic therapy to range from 5-78% and "clinically significant' elevations in 0-15%; lists 1 report of clinically apparent liver injury due to ziprasidone [Tsai 2005]).
- Findling RL, Cavuş I, Pappadopulos E, Vanderburg DG, Schwartz JH, Gundapaneni BK, Delbello MP. Efficacy, long-term safety, and tolerability of ziprasidone in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2013;23:545–57. [PMC free article: PMC3804078] [PubMed: 24111980](Controlled trial of ziprasidone vs placebo for 4 weeks with a 26 week open label extension study in 237 children or adolescents with bipolar disorder found no "clinically significant mean changes" in liver enzymes).
- Drugs for psychiatric disorders. Treat Guidel Med Lett. 2013;11(130):53–64. [PubMed: 23715100](Concise summary of current therapies for psychiatric disorders, mentions that ziprasidone can cause prolongation of QT interval and extrapyramidal symptoms, but does not mention liver injury or ALT elevations).
- Kim MS, Kim SW, Han TY, Son SJ, Lee JH, Kim EJ. Ziprasidone-induced hypersensitivity syndrome confirmed by reintroduction. Int J Dermatol. 2014;53:e267–8. [PubMed: 24117439](33 year old Korean woman developed rash and fever 3 weeks after starting ziprasidone and lithium, with resolution of stopping both and recurrence one day after restarting ziprasidone [ALT 141, atypical lymphocytes 12%], resolving within 2 weeks of stopping).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, only one of which was attributed to chlorpromazine, the only antipsychotic medication listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 cases [0.6%] were attributed to antipsychotic agents, including 3 due to quetiapine and 2 to olanzapine, but none to ziprasidone]).
- Mandrioli R, Protti M, Mercolini L. Evaluation of the pharmacokinetics, safety and clinical efficacy of ziprasidone for the treatment of schizophrenia and bipolar disorder. Expert Opin Drug Metab Toxicol. 2015;11:149–74. [PubMed: 25483358](Review of the efficacy and safety of ziprasidone does not discuss ALT elevations or hepatotoxicity).
- Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14:73–96. [PubMed: 25400109](Extensive systematic review of the literature on the problem of weight gain during therapy with antipsychotic agents mentions that weight gain of 7% or more occurs in only 4% of patients on ziprasidone and the average weight change is -0.16 to 0.84 kg, rates of weight gain being lower than any other atypical antipsychotic).
- Morlán-Coarasa MJ, Arias-Loste MT, Ortiz-García de la Foz V, Martínez-García O, Alonso-Martín C, Crespo J, Romero-Gómez M, et al. Incidence of non-alcoholic fatty liver disease and metabolic dysfunction in first episode schizophrenia and related psychotic disorders: a 3-year prospective randomized interventional study. Psychopharmacology (Berl). 2016;233:3947–52. [PubMed: 27620899](Among 205 patients started on atypical antipsychotic agents between 2005 and 2012 followed using surrogate markers for fatty liver disease and for fibrosis [Fib-4], 48 [25%] patients developed evidence of fatty liver disease as assessed by FLI, driven largely by weight gain [91%] and increases in waist circumference [69%] and triglycerides [54%]; analysis by specific agent was not provided, most were taking aripiprazole [n=83], risperidone [n=12], quetiapine [n=46] or ziprasidone [50]).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that olanzapine can cause aminotransferase elevations, and that olanzapine and ziprasidone can cause DRESS syndrome, but does not mention ALT elevations or hepatotoxicity for any of agents discussed, including aripiprazole, brexpiprazole, cariprazine, clozapine, quetiapine, risperidone, asenapine, iloperidone, paliperidone and lurasidone).
- Patel MB, Maddur H. Elevated levels of AST, ALT, and CPK · no family history of liver disease · Dx? J Fam Pract. 2017;66:450–2. [PubMed: 28700759](25 year old man was found to have marked serum enzyme elevations two years after starting valproate and ziprasidone [ALT 334 U/L, AST 1040 U/L, CPK 34,270] and a month after starting a muscle building agent “Code Red” containing DMAA, resolving rapidly with stopping the supplement and interpreted as rhabdomyolysis due to DMAA).
- Schreiner NM, Windham S, Barker A. Atypical neuroleptic malignant syndrome: diagnosis and proposal for an expanded treatment algorithm: a case report. A A Case Rep. 2017;9:339–43. [PubMed: 28767476](48 year old man with bipolar disorder and NASH underwent liver transplantation and developed confusion, dyskinesia, rigidity, hyperthermia and tachycardia/tachypnea postoperatively having been given lithium, lamotrigine, promethazine and ziprasidone, responding to therapy of neuroleptic malignant syndrome with benzodiazepines and propofol).
- Baeza I, de la Serna E, Calvo-Escalona R, Merchán-Naranjo J, Rodríguez-Latorre P, Martínez-Cantarero MC, Andrés P, et al. One-year prospective study of liver function tests in children and adolescents on second-generation antipsychotics: is there a link with metabolic syndrome? J Child Adolesc Psychopharmacol. 2018;28:463–73. [PubMed: 29975563](Among 216 children and adolescents starting atypical antipsychotics, mean weight gain at 6 months was 6.5 kg and mean ALT levels increased by 8.6 U/L, while among 37 taking olanzapine mean weight gain was 10.3 kg and ALT increase 2.6 U/L; increases in ALT associated with development of the metabolic syndrome, mean ALT increasing by 27.8 U/L at 6 months; results using ziprasidone not included).
- Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, et al. MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506–2516. [PMC free article: PMC6364999] [PubMed: 30346242](Among 566 adults who developed delirium during therapy for respiratory failure or shock and were treated with ziprasidone [maximum dose 40 mg daily], haloperidol [maximum dose 20 mg daily], or placebo, duration of delirium and major outcomes were similar in the three groups; no mention of ALT elevations or hepatotoxicity but QTc prolongation was more frequent with ziprasidone).
- Druschky K, Toto S, Bleich S, Baumgärtner J, Engel RR, Grohmann R, Maier HB, et al. Severe drug-induced liver injury in patients under treatment with antipsychotic drugs: data from the AMSP study. World J Biol Psychiatry. 2021;22:373–386. [PubMed: 32892689](Among 246 cases of severe liver injury due to antipsychotic medications identified in a prospective registry of German psychiatric hospitals between 1993 and 2016, 46 arose in 38,349 patients [0.12%] who received clozapine [34 as a single antipsychotic agent]; other commonly implicated agents being olanzapine [n=90 of 54,822: 0.16%], quetiapine [34 of 66,209: 0.05%] and risperidone [27 of 51,683: 0.05%]; two fatal cases occurred in olanzapine-treated patients; low rates were found for ziprasidone [no cases among 3568 patients treated] and aripiprazole [6 cases of 15,988 patients treated: 0.01%).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine, but none for risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine).
- Atkinson S, Bachinsky M, Raiter Y, Abreu P, Ianos C, Chappell P, Findling RL. 26-Week open-label extension study evaluating the safety and tolerability of flexible doses of oral ziprasidone in children and adolescents with bipolar I disorder (Most Recent Episode Manic). J Child Adolesc Psychopharmacol. 2022;32:453–458. [PubMed: 36282771](Among 23 adolescents treated with ziprasidone in a 4 week controlled trial who were started or continued on therapy for up to 26 weeks, side effects included fatigue, somnolence, nausea and extrapyramidal symptoms, usually transiently shortly after starting; no mention of ALT levels or hepatotoxicity).
- Gunther M, Dopheide JA. Antipsychotic safety in liver disease: a narrative review and practical guide for the clinician. J Acad Consult Liaison Psychiatry. 2023;64:73–82. [PubMed: 36180017](Review of the literature on hepatotoxicity of antipsychotic medications and guidance on their use in patients with liver disease characterizes chlorpromazine, clozapine, and olanzapine as having the greatest risk for causing liver injury, quetiapine and risperidone as having moderate risk, haloperidol as having low risk and paliperidone, aripiprazole, lurasidone, loxapine, and ziprasidone as having low risk).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Ziprasidone in the treatment of mania in bipolar disorder.[Neuropsychiatr Dis Treat. 2007]Ziprasidone in the treatment of mania in bipolar disorder.Nicolson SE, Nemeroff CB. Neuropsychiatr Dis Treat. 2007 Dec; 3(6):823-34.
- Review Ziprasidone: an atypical antipsychotic drug for the treatment of schizophrenia.[Clin Ther. 2002]Review Ziprasidone: an atypical antipsychotic drug for the treatment of schizophrenia.Stimmel GL, Gutierrez MA, Lee V. Clin Ther. 2002 Jan; 24(1):21-37.
- Review Ziprasidone for schizophrenia and bipolar disorder: a review of the clinical trials.[CNS Drug Rev. 2007]Review Ziprasidone for schizophrenia and bipolar disorder: a review of the clinical trials.Greenberg WM, Citrome L. CNS Drug Rev. 2007 Summer; 13(2):137-77.
- Review Ziprasidone, a new atypical antipsychotic drug.[Pharmacotherapy. 2001]Review Ziprasidone, a new atypical antipsychotic drug.Carnahan RM, Lund BC, Perry PJ. Pharmacotherapy. 2001 Jun; 21(6):717-30.
- Review Ziprasidone: the fifth atypical antipsychotic.[Ann Pharmacother. 2002]Review Ziprasidone: the fifth atypical antipsychotic.Caley CF, Cooper CK. Ann Pharmacother. 2002 May; 36(5):839-51.
- Ziprasidone - LiverToxZiprasidone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...