NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Zafirlukast is an orally available leukotriene receptor antagonist which is widely used for the prophylaxis and chronic treatment of asthma. Zafirlukast has been linked to rare, but occasionally severe cases of acute liver injury.
Background
Zafirlukast (za" fir loo' kast) is a leukotriene receptor antagonist that binds to the CysLT1 and CysLT2 receptors, thereby interfering with inflammatory pathways that are involved in the pathogenesis of asthma and allergic rhinitis. Pretreatment with single oral doses of zafirlukast inhibits bronchoconstriction and the early-phase and part of the late-phase inflammatory reactions in asthma. Zafirlukast was approved for use in the treatment of asthma in the United States in 1996, and it continues to be widely used with more than 2 million prescriptions filled yearly. Other leukotriene receptor antagonists include montelukast and pranlukast, but only montelukast is also available in the United States. Current indications include the prophylaxis and chronic treatment of asthma in adults and children (above the age of 5). Zafirlukast is available in 10 and 20 mg tablets generically and under the brand name Accolate. The recommended dosage of zafirlukast in adults and children >12 years is 20 mg twice daily. The recommended dose in children 5 to 11 years is 10 mg twice daily. Common side effects include nausea, diarrhea, dyspepsia, abdominal pain, headache, dizziness, myalgias, and fever.
Hepatotoxicity
Prospective studies have shown that ALT elevations occur in 1.5% of patients receiving zafirlukast, most of which are mild, asymptomatic and self limited even with continuing therapy. A similar rate of transient ALT elevations is often found in placebo recipients. Clinically apparent liver disease from zafirlukast is rare, but many convincing cases have been reported, several of which were severe and resulted in hepatic failure, need for liver transplantation or death. The onset of symptoms of liver injury is typically within 2 to 6 months of starting therapy, but cases with longer latency periods have been reported (8-13 months). Symptoms include fatigue, nausea, and right upper quadrant pain followed by dark urine, jaundice and pruritus. The pattern of liver enzyme elevation is usually hepatocellular and resembles acute viral hepatitis. Eosinophilia is common but immunoallergic features are usually not prominent and autoantibodies are uncommon (eosinophilia may be due to the underlying allergic asthmatic condition). Fatal cases have been described. Reexposure can lead to more rapid and severe reappearance of injury and should be avoided. Zafirlukast can also cause systemic vasculitis and eosinophilia (Churg-Strauss Syndrome) which may be accompanied by hepatic involvement and mild enzyme elevations.
Likelihood score: C (probable cause of clinical apparent liver injury).
Mechanism of Hepatotoxicity
The mechanism of hepatic injury due to zafirlukast is not known but it is clearly idiosyncratic. The extensive hepatic metabolism of zafirlukast by the cytochrome P450 (CYP2C9) system suggests that injury may be a result of a hepatotoxic or immunogenic intermediate.
Outcome and Management
Monitoring of routine liver tests for liver injury is recommended but has not been shown to be effective in preventing the rare instances of hepatic injury due to zafirlukast and the optimal timing and frequency of such testing is not well define. Patients should be alerted to the signs and symptoms of liver injury and stop therapy or be seen quickly if they occur. Typically, symptoms and liver enzymes abnormalities resolve rapidly after stopping zafirlukast. In rare cases, patients have either presented with fulminant hepatitis or progressed to hepatic failure, liver transplantation and death. Rechallenge leads to prompt recurrence and should be avoided. Switching to another leukotriene receptor antagonist such as montelukast or to zileuton, a 5-lipoxygenase inhibitor with a related mechanism of action, is usually possible but should be done with caution.
Drug Class: Antiasthmatic Agents
Other Drugs in the Subclass, Leukotriene Receptor Antagonists: Montelukast
CASE REPORTS
Case 1. Acute hepatitis related to zafirlukast use.
[Modified from: Moles JM, Primo J, Fernandez JM, Hinojosa JE. Acute hepatocellular injury associated with zafirlukast. J Hepatol 2001; 35: 541-2. PubMed Citation]
A 63 year old woman with asthma developed fatigue and anorexia 8 weeks after adding zafirlukast (20 mg twice daily) to a chronic regimen of various inhalants including salbutamol, fomoterol and budesonide. Two weeks later she noted dark urine and jaundice, but waited another two weeks before seeking medical advice. She had no history of liver disease and serum aminotransferase levels had been normal in the past. She had no risk factors for viral hepatitis, did not use alcohol, and took no other medications. Her past medical history included gall bladder disease leading to a cholecystectomy 4 years previously. On examination, she was jaundiced but had no fever, rash, hepatomegaly or signs of chronic liver disease. Laboratory tests showed a total bilirubin of 4.5 mg/dL, ALT 999 U/L, AST 980 U/L, alkaline phosphatase 323 U/L and GGT 258 (normal <50) U/L. The prothrombin time was normal and tests for acute hepatitis A, B and C were negative as were autoantibodies. An abdominal ultrasound was normal. Zafirlukast was discontinued on admission and she improved rapidly (Table). Liver biopsy was not done. Symptoms and jaundice resolved within 2 weeks and liver tests were normal when she was seen 3 months after stopping.
Key Points
| Medication: | Zafirlukast (20 mg twice daily) |
| Pattern: | Hepatocellular (R=19.2) |
| Severity: | 3+ (hospitalization for jaundice) |
| Latency: | 8 weeks to symptoms, 10 weeks to jaundice |
| Recovery: | 3 months |
| Other medications: | Salmeterol and fomoterol aerosol and budesonide inhaler |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 8 weeks | Anorexia and fatigue | ||||
| 10 weeks | Dark urine and jaundice | ||||
| 12 weeks | 0 | 999 | 323 | 4.5 | Zafirlukast stopped |
| 13 weeks | 7 days | 918 | 683 | 2.6 | Normal ultrasound |
| 14 weeks | 18 days | 560 | 729 | 1.6 | |
| 5 months | 2 months | 130 | 303 | 1.0 | Asymptomatic |
| 6 months | 3 months | 21 | 249 | 0.6 | |
| 9 months | 6 months | 27 | 260 | 0.7 | |
| Normal Values | <40 | <280 | <1.2 | ||
Comment
This patient developed an acute hepatitis-like syndrome 2 months after starting zafirlukast for chronic asthma, which began to resolve within a week of stopping the suspected agent. She was taking no other medications except inhalants which she had used for years, and clinical evaluation ruled out other common causes of liver injury. Zafirlukast has been linked to several instances of acute liver injury usually arising within 2 to 6 months of starting and presenting with jaundice and a hepatocellular pattern of liver injury. The course is usually self limiting, but several instances of acute liver failure leading to death or emergency liver transplantation have been published.
Case 2. Acute liver injury due to zafirlukast.
[Modified from: Reinus JF, Persky S, Burkiewicz JS, Quan D, Bass NM, Davern TJ. Severe liver injury after treatment with the leukotriene receptor antagonist zafirlukast. Ann Intern Med 2000; 133: 964-8. PubMed Citation]
A 42 year old woman with chronic sinusitis and asthma was found to have abnormal serum enzymes 9 months after she was started on zafirlukast. She had no symptoms, but therapy was stopped and she was switched to montelukast, on which her serum aminotransferase levels fell to normal. Zafirlukast was restarted, but 2 months later she developed symptoms and jaundice. When seen one month later, she was jaundiced and had marked serum aminotransferase elevations (Table). She had no history of exposure to viral hepatitis and did not drink alcohol. She recovered slowly, jaundice resolved within 2 months and serum enzyme elevations within 5 months of stopping.
Key Points
| Medication: | Zafirlukast (20 mg twice daily) |
| Pattern: | Hepatocellular |
| Severity: | 3+ (hospitalization for jaundice) |
| Latency: | Initially, 9 months, 2 months on rechallenge |
| Recovery: | 5 months on rechallenge |
| Other medications: | Salmeterol aerosol and fluticasone propionate inhaler and nasal spray |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Started zafirlukast 20 mg twice daily | |||||
| 9 months | 0 | 295 | 0.7 | Started montelukast | |
| 13 months | 4 months | Normal | 0.7 | ||
| Resumed zafirlukast 20 mg twice daily | |||||
| 2 months | 0 | Symptoms and jaundice: zafirlukast stopped | |||
| 3 months | 1 month | 1566 | 6.2 | ||
| 4 months | 2 months | 270 | 3.1 | ||
| 5 months | 3 months | 1.4 | |||
| 6 months | 4 months | 1.2 | |||
| 7 months | 5 months | Normal | 0.5 | Asymptomatic | |
| Normal Values | <35 | <130 | <1.2 | ||
* Selected values estimated from Figure.
Comment
The initial ALT elevations had several possible explanations, but the reappearance of hepatocellular injury upon rechallenge is firm evidence of the role of zafirlukast. Interestingly, she tolerated montelukast, another leukotriene receptor antagonist with a somewhat similar chemical structure, without evidence of liver injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Zafirlukast – Generic, Accolate®
DRUG CLASS
Antiasthma Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Zafirlukast | 107753-78-6 | C31-H33-N3-O6-S |
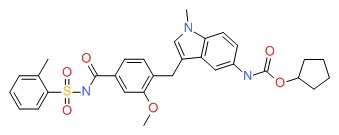 |
ANNOTATED BIBLIOGRAPHY
References updated: 04 June 2019
- Zimmerman HJ. Respiratory supportive drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, p. 717.(Expert review of hepatotoxicity published in 1999; mentions that theophylline has been incriminated in hepatic injury rarely; no mention of montelukast, zafirlukast or zileuton).
- Lewis JH. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013. pp. 389-90.(Expert review of liver injury caused by leukotriene receptor antagonists mentions that there were no reports of clinically apparent liver injury during premarketing studies of zafirlukast, but that more than 100 reports of clinically apparent injury appeared thereafter, at least 14 resulting in liver failure).
- Barnes PJ. Pulmonary pharmacology. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 727-30.(Textbook of pharmacology and therapeutics).
- Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 1999; 340: 197-206. [PubMed: 9895400](Review article on leukotriene pathway in asthma and rhinitis and efficacy and safety of montelukast, zafirlukast and zileuton; mentions that abnormal ALT elevations can occur).
- Gozalo Reques F, Estrada Rodriguez JL. Tolerability of leukotriene modifiers in asthma: a review of clinical experience. Biodrugs 1999; 11: 385-94. [PubMed: 18031150](Review of safety of leukotriene antagonists without new information).
- Grossman J, Smith LJ, Wilson AM, Thyrum PT. Long-term safety and efficacy of zafirlukast in the treatment of asthma: interim results of an open-label extension trial. Ann Allergy Asthma Immunol 1999; 82: 361-9. [PubMed: 10227334](Open labeled continuation of controlled trial of zafirlukast for 39 weeks; among 443 patients, 4.4% had ALT elevations, but peak value 340 U/L and all were self limited; one patient was withdrawn because of ALT elevations).
- Grieco AJ, Burstein-Stein J. Oral montelukast versus inhaled salmeterol to prevent exercise-induced bronchoconstriction. Ann Intern Med 2000; 133: 392. [PubMed: 10979888](68 year old woman developed jaundice 4 months after starting zafirlukast [bilirubin 21.1 mg/dL, ALT 1217 U/L, Alk P 225 U/L], resolving upon stopping).
- Reinus JF, Persky S, Burkiewicz JS, Quan D, Bass NM, Davern TJ. Severe liver injury after treatment with the leukotriene receptor antagonist zafirlukast. Ann Intern Med 2000; 133: 964-8. [PubMed: 11119397](Three patients developed jaundice 5, 6 and 9 months after starting zafirlukast [peak bilirubin 6.2, 39.1 and 20.3 mg/dL, ALT 1750, 1157, and 1212 U/L, Alk P not given], one patient developed acute liver failure and 2 recovered within 4 months of stopping: Case 2).
- Actis GC, Morgando A, Lagget M, David E, Rizzetto M. Zafirlukast-related hepatitis: report of a further case [Letter]. J Hepatol 2001; 35: 539-41. [PubMed: 11682043](54 year old man developed jaundice 8 months after starting zafirlukast [bilirubin 3.9 mg/dL, ALT 661, and Alk P 135 U/L] with prolonged course despite corticosteroids, histology revealed severe injury with collapse).
- Danese S, De Vitis I, Gasbarrini A. Severe liver injury associated with zafirlukast [Letter]. Ann Intern Med 2001; 135: 930. [PubMed: 11712893](55 year old woman developed severe hepatitis 5 months after starting zafirlukast [bilirubin not given, ALT 2113 U/L, GGT 15 times ULN], there was a delay in stopping therapy and liver biopsy showed submassive necrosis and recovery was slow).
- Molés JR, Primo J, Fernández JM, Hinojosa JE. Acute hepatocellular injury associated with zafirlukast. J Hepatol 2001; 35: 541-2. [PubMed: 11682044](63 year old woman developed jaundice 2 months after starting zafirlukast [bilirubin 4.5 mg/dL, ALT 999 U/L, Alk P 683 U/L, GGT 258 U/L], resolving within 3 months of stopping: Case 1).
- Spector SL; Antileukotriene Working Group. Safety of antileukotriene agents in asthma management. Ann Allergy Asthma Immunol 2001; 86 (6 Suppl 1): 18-23. [PubMed: 11426912](Review of side effects of antileukotrienes in premarketing studies, ALT elevations found in 1.4% of subjects on zafirlukast vs 1.1% on placebo; in 2.1% on montelukast vs 2.0% on placebo; and in 4.6% of zileuton recipients treated for a year [>3 times ULN; 3 had hyperbilirubinemia] vs 1.1% of controls).
- Torres M, Reddy KR. Severe liver injury. Ann Intern Med. 2001; 135: 550. [PubMed: 11578163](36 year old woman developed jaundice 13 months after starting zafirlukast [peak bilirubin 28 mg/dL, ALT 3135 U/L, Alk P not given], followed by liver failure and emergency liver transplantation).
- Soy M, Ozer H, Canataroglu A, Gumurdulu D, Erken E. Vasculitis induced by zafirlukast therapy. Clin Rheumatol 2002; 21: 328-9. [PubMed: 12189465](54 year old man developed purpura, malaise, pleural effusions and atectasis 2 weeks after starting zafirlukast [bilirubin normal, ALT 98 U/L, Alk P 780 U/L, 35% eosinophils], improving upon stopping drug and use of corticosteroids, felt to be Churg-Straus syndrome).
- Su CW, Wu JC, Huang YH, Huang YS, Chang FY, Lee SD. Zafirlukast-induced acute hepatitis. Zhonghua Yi Xue Za Zhi(Taipei) 2002; 65: 553-6. [PubMed: 12583521](69 year old woman developed jaundice 3 months after starting zafirlukast [bilirubin 18.2 rising to 34.8 mg/dL, ALT 481 U/L, Alk P 346 U/L], resolving within 3 months of stopping).
- Davern TJ, Bass NM. Leukotriene antagonists. Clin Liver Dis 2003; 7: 501-12, viii. [PubMed: 12879996](Review of literature on hepatotoxicity of leukotriene inhibitors; in premarketing studies, 1.5% of 4058 of zafirlukast vs 1.1% of placebo recipients had ALT elevations, none serious and all transient; review of literature identified 9 cases of zafirlukast hepatotoxicity, 7 with jaundice, 3 with rash and eosinophilia, arising after 1.5 to 13 months, with liver transplantation required in two).
- Wooltorton E. Asthma drug zafirlukast (Accolate): serious hepatic events. CMAJ 2004; 170: 1668. [PMC free article: PMC408503] [PubMed: 15159361](Editorial on zafirlukast and its linkage to serious liver injury).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, one case was attributed to zafirlukast, but no other antiasthma medications mentioned).
- Chohnabayashi N, Sugiura R, Nishimura N. [Adverse effects of leukotriene-antagonists] Nippon Rinsho 2007; 65 Suppl 8: 272-6. Japanese. [PubMed: 18072343](Review article on adverse events of leukotriene antagonists including montelukast, zafirlukast and pranlukast).
- Twaites BR, Wilton LV, Shakir SA. Safety of zafirlukast. Results of a postmarketing surveillance study on 7976 patients in England. Drug Saf 2007; 30: 419-29. [PubMed: 17472420](Database of UK medical practice with questionnaire requesting information on zafirlukast treated patients, 7976 forms returned from 21,557 requests; 61% of physicians considered it effective, 53% of patients were treated for >6 months; 152 adverse events were reported in 120 patients [1.5%] including 34 hepatic events [4 with jaundice, 2 with hepatitis], but none were fatal and the authors concluded that “none of the events were serious”).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to an antiasthma medication).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 1 due to zafirlukast, but none to montelukast or zileuton).
- Agarwal VK, McHutchison JG, Hoofnagle JH; Drug-Induced Liver Injury Network. Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol 2010; 8: 463-70. [PMC free article: PMC3901223] [PubMed: 20170750](Review of the completeness of documentation of drug induced liver disease in published case reports of 6 different drugs [including 8 reports of zafirlukast and 4 of montelukast hepatotoxicity] identified important missing data in most reports).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-Induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to zafirlukast or other antiasthma medications).
- Aagaard L, Hansen EH. Adverse drug reactions associated with asthma medications in children: systematic review of clinical trials. Int J Clin Pharm 2014; 36: 243-52. [PubMed: 24562976](Systematic review of adverse events reported in controlled trials of asthma medications including zafirlukast in children; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, montelukast was implicated in 4 cases, but zafirlukast and other antiasthma agents were not implicated in any cases).
- Leung JS, Johnson DW, Sperou AJ, Crotts J, Saude E, Hartling L, Stang A. A systematic review of adverse drug events associated with administration of common asthma medications in children. PLoS One 2017; 12: e0182738. [PMC free article: PMC5549998] [PubMed: 28793336](Systematic review of adverse events in reported in 46 clinical studies of commonly used asthma medications including 25 events associated with leukotriene antagonists affecting 9 organ systems; hepatotoxicity not mentioned).
- Drugs for asthma. Med Lett Drugs Ther 2017; 59 (1528): 139-146. [PubMed: 28880849](Concise review of medications used for asthma including the leukotriene modifiers; mentions that zafirlukast and zileuton have been reported to cause life-threatening hepatic injury and that monitoring of liver tests during therapy is recommended).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Zafirlukast: the first leukotriene-receptor antagonist approved for the treatment of asthma.[Ann Pharmacother. 1997]Review Zafirlukast: the first leukotriene-receptor antagonist approved for the treatment of asthma.Kelloway JS. Ann Pharmacother. 1997 Sep; 31(9):1012-21.
- Review Zafirlukast. A review of its pharmacology and therapeutic potential in the management of asthma.[Drugs. 1998]Review Zafirlukast. A review of its pharmacology and therapeutic potential in the management of asthma.Adkins JC, Brogden RN. Drugs. 1998 Jan; 55(1):121-44.
- Severe liver injury after treatment with the leukotriene receptor antagonist zafirlukast.[Ann Intern Med. 2000]Severe liver injury after treatment with the leukotriene receptor antagonist zafirlukast.Reinus JF, Persky S, Burkiewicz JS, Quan D, Bass NM, Davern TJ. Ann Intern Med. 2000 Dec 19; 133(12):964-8.
- Summary of clinical trials with zafirlukast.[Am J Respir Crit Care Med. 1998]Summary of clinical trials with zafirlukast.Calhoun WJ. Am J Respir Crit Care Med. 1998 Jun; 157(6 Pt 1):S238-46.
- Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast.[Manag Care Interface. 2001]Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast.Klingman D, Bielory L, Wang Y, Silverman S, Bell CF, Joy KA, Dever MT, Jones DA. Manag Care Interface. 2001 Feb; 14(2):62-6.
- Zafirlukast - LiverToxZafirlukast - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...