NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Vorinostat is an oral histone deacetylase inhibitor and antineoplastic agent that is approved for use in refractory or relapsed cutaneous T cell lymphoma. Vorinostat is associated with modest rate of minor serum enzyme elevations during therapy, but has not been linked to cases of clinically apparent liver injury.
Background
Vorinostat (vor in' oh stat) is an oral small molecule inhibitor of histone deacetylase which acts by preventing removal of acetyl groups from histones. The accumulation of acetyl groups on histones causes cell cycle arrest and apoptotic cell death. Malignant cells and particularly malignant T cells are particularly sensitive to the effects of inhibition of histone deacetylases. In open label studies in patients with refractory cutaneous T cell lymphoma (CTCL), monotherapy with vorinostat yielded an overall response rate of 30%, and some responders had long term remissions and were able to undergo hematopoietic cell transplantation. Vorinostat has also been evaluated in B cell lymphomas and in several forms of solid tumors, but with only modest results. Vorinostat was approved for use in the United States in 2006 as monotherapy for refractory or relapsing cutaneous T cell lymphoma, the first histone deacetylase inhibitor approved as an anticancer agent. Vorinostat is available in capsules of 100 mg under the commercial name Zolinza. The recommended dose is 400 mg daily by mouth, continuing therapy until there is disease progression or unacceptable toxicity. Side effects are common, but usually mild-to-moderate in severity, and include nausea, fatigue, fever, anemia, neutropenia, thrombocytopenia, diarrhea, constipation, rash, edema, cough and pruritus. Side effects lead to early discontinuation in up to 8% of patients. Severe adverse events can include marked neutropenia, thrombocytopenia, serious infections, sepsis, tumor lysis syndrome and embryo-fetal toxicity.
Hepatotoxicity
In clinical trials of vorinostat in patients with CTCL, the rates of serum enzyme elevations during therapy were rarely mentioned and only occasional episodes of mild elevations were recorded. Minor elevations in serum ALT levels occurred in 15% to 45% of patients, but values above 5 times ULN were rare and there were no reports of hepatitis, jaundice or clinically apparent liver injury among the treated subjects. Vorinostat has had limited clinical use, but there have been no published reports of its association with significant liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The reason why vorinostat might cause serum enzyme elevations is not known, but may be a direct toxicity to hepatocytes caused by inhibition of histone deacetylase or other enzyme activities. Vorinostat is metabolized in the liver by glucuronidation and is not a substrate, inhibitor or inducer of cytochrome P450 enzymes.
Outcome and Management
Serum enzyme elevations can occur during vorinostat therapy, but they are usually transient and mild-to-moderate in severity, rarely requiring dose modification. There is no known cross sensitivity to hepatic injury among the different histone deacetylase inhibitors.
Drug Class: Antineoplastic Agents, Histone Deacetylase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Vorinostat – Zolinza®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
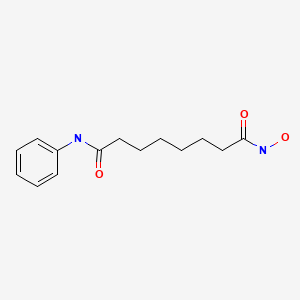
| Vorinostat | 149647-78-9 | C14-H20-N2-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 12 September 2020
- Abbreviation: CTCL, cutaneous T-cell lymphoma.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of histone deacetylase inhibitors).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; does not discuss vorinostat).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Inhibitors of histone deacetylase. Pathway targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, p. 1230.(Textbook of pharmacology and therapeutics).
- Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–31. [PMC free article: PMC1855284] [PubMed: 15897550](Among 73 patients with different forms of advanced cancer treated with several regimens of vorinostat, the maximum tolerated dose was 400 mg daily and toxicities included fatigue, anorexia, nausea, diarrhea, bone marrow suppression, hyperglycemia, creatinine elevations and proteinuria; no mention of ALT elevations).
- Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–15. [PubMed: 17577020](Among 74 patients with refractory or relapsed CTCL treated with vorinostat, the overall response rate was 30% and adverse events included diarrhea [49%], fatigue [46%], nausea [43%] and anorexia [26%]; no mention of ALT elevations or hepatotoxicity).
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109:31–9. [PMC free article: PMC1785068] [PubMed: 16960145](Among 33 patients with refractory or relapsed CTCL treated with vorinostat, the overall response rate was 24% and side effects were common, serious adverse events being dehydration, thrombocytopenia, vomiting, anemia, neutropenia and infections; no mention of ALT elevations or hepatotoxicity).
- Vorinostat (Zolinza) for cutaneous T-Cell lymphoma. Med Lett Drugs Ther. 2007;49(1256):23–4. [PubMed: 17351559](Concise review of the mechanism of action, clinical efficacy, safety and costs of vorinostat shortly after its approval for use in the US; mentions serious adverse events of pulmonary embolism, squamous cell carcinoma of skin and fetal abnormalities).
- Crump M, Coiffier B, Jacobsen ED, Sun L, Ricker JL, Xie H, Frankel SR, et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann Oncol. 2008;19:964–9. [PubMed: 18296419](Among 18 patients with relapsed B cell lymphoma treated with vorinostat, the overall response rate was 5% [1 patient] and serious adverse events occurred in 7 patients [39%], mostly hematologic and gastrointestinal events; no mention of ALT elevations or hepatotoxicity).
- Duvic M, Olsen EA, Breneman D, Pacheco TR, Parker S, Vonderheid EC, Abuav R, et al. Evaluation of the long-term tolerability and clinical benefit of vorinostat in patients with advanced cutaneous T-cell lymphoma. Clin Lymphoma Myeloma. 2009;9:412–6. [PubMed: 19951879](Six of the 74 patients with CTCL enrolled in a trial of vorinostat were treated for 2 years, all with only moderate toxicities).
- Traynor AM, Dubey S, Eickhoff JC, Kolesar JM, Schell K, Huie MS, Groteluschen DL, et al. Vorinostat (NSC# 701852) in patients with relapsed non-small cell lung cancer: a Wisconsin Oncology Network phase II study. J Thorac Oncol. 2009;4:522–6. [PMC free article: PMC3050710] [PubMed: 19347984](Among 18 patients with relapsed NSCLC treated with vorinostat, there were no partial or complete response and side effects were common; no mention of ALT elevations or hepatotoxicity).
- Kavanaugh SM, White LA, Kolesar JM. Vorinostat: A novel therapy for the treatment of cutaneous T-cell lymphoma. Am J Health Syst Pharm. 2010;67:793–7. [PubMed: 20479100](Review of the mechanism of action, pharmacology, clinical efficacy and safety of vorinostat therapy of cutaneous T cell lymphoma mentions an overall response rate of 30% and frequency of side effects including fatigue in 52%, nausea in 41%, but no mention of ALT elevations or hepatotoxicity).
- Kirschbaum M, Frankel P, Popplewell L, Zain J, Delioukina M, Pullarkat V, Matsuoka D, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29:1198–203. [PMC free article: PMC3083875] [PubMed: 21300924](Among 35 patients with non-Hodgkin lymphoma treated with vorinostat for an average of 6 months, overall response rates were 29% in follicular lymphoma, but 0% with mantle cell lymphoma; ALT or AST elevations occurred in 2 patients [3%], but both were transient and less than 3 times ULN).
- Kirschbaum MH, Goldman BH, Zain JM, Cook JR, Rimsza LM, Forman SJ, Fisher RI. A phase 2 study of vorinostat for treatment of relapsed or refractory Hodgkin lymphoma: Southwest Oncology Group Study S0517. Leuk Lymphoma. 2012;53:259–62. [PMC free article: PMC3477846] [PubMed: 21823829](Among 25 patients with refractory or relapsed Hodgkin lymphoma treated with vorinostat for an average of 3.8 months, the overall response rate was 8%; no mention of ALT elevations or hepatotoxicity).
- Ogura M, Ando K, Suzuki T, Ishizawa K, Oh SY, Itoh K, Yamamoto K, et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol. 2014;165:768–76. [PMC free article: PMC4282031] [PubMed: 24617454](Among 39 patients with follicular lymphoma treated with vorinostat, the overall response rate was 39%; adverse events were common, but no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 363 [36%] were attributed to antibiotics, none of which were attributed to vorinostat).
- Krug LM, Kindler HL, Calvert H, Manegold C, Tsao AS, Fennell D, Öhman R, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2015;16:447–56. [PubMed: 25800891](Among 661 patients with advanced mesothelioma treated with vorinostat or placebo, median overall survival was not improved by vorinostat, which had higher rates of adverse events including nausea, vomiting, diarrhea, anorexia, weight loss, and fever while ALT elevations were uncommon [1% vs none]).
- Duvic M, Dimopoulos M. The safety profile of vorinostat (suberoylanilide hydroxamic acid) in hematologic malignancies: A review of clinical studies. Cancer Treat Rev. 2016;43:58–66. [PubMed: 26827693](Review of the adverse event rates in various trials of vorinostat and comparison to other histone deacetylase inhibitors reported a high rate of adverse events that were serious and drug related in 15% of patients and led to drug discontinuation in 8%; the most common side effects were diarrhea, nausea, fatigue and thrombocytopenia; only rare and occasional ALT elevation noted and no mention of hepatotoxicity or clinically apparent liver injury).
- Schmitt T, Mayer-Steinacker R, Mayer F, Grünwald V, Schütte J, Hartmann JT, Kasper B, et al. Vorinostat in refractory soft tissue sarcomas - Results of a multi-centre phase II trial of the German Soft Tissue Sarcoma and Bone Tumour Working Group (AIO). Eur J Cancer. 2016;64:74–82. [PubMed: 27367154](Open label study of vorinostat in 40 patients with advanced soft tissue sarcoma found a low response rate while adverse events were largely hematologic [15%], gastrointestinal [13%], fatigue {10%] and musculoskeletal pain [10%]; no mention of ALT elevations or hepatotoxicity).
- Bringhen S, De Wit E, Dimopoulos MA. New agents in multiple myeloma: an examination of safety profiles. Clin Lymphoma Myeloma Leuk. 2017;17:391–407.e5. [PubMed: 28601492](Review of the safety profiles and adverse events of newer drugs for multiple myeloma including the histone deacetylase inhibitors – romidepsin, vorinostat and panobinostat, mentions that ALT elevations above 5 times ULN occur in 8% of patients treated with romidepsin, but no mention of ALT elevations or hepatotoxicity with vorinostat or panobinostat).
- Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma. 2017;58:1306–19. [PubMed: 27813438](Extensive review of the mechanism of action, classification, clinical efficacy and safety of histone deacetylase inhibitors in T-cell lymphomas, mentions that vorinostat is an oral hydroxamate that is approved for use in refractory or relapsed cutaneous T cell lymphoma, more common adverse effects of which are fatigue, diarrhea, nausea, dysgeusia, thrombocytopenia, anorexia, weight loss and muscle spasms; no mention of ALT elevations or hepatotoxicity).
- Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, Whittaker S, et al. MAVORIC Investigators. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192–204. [PubMed: 30100375](Among 372 adults with refractory or relapsed mycosis fungoides or Sezary syndrome treated with either vorinostat or mogamulizumab [monoclonal anti-C-C chemokine receptor 4], median progression free survival was less with vorinostat [3.1 vs 7.7 months], while adverse event rates were similar including AST elevations [6.5% vs 4.35]; no mention of hepatotoxicity or clinically apparent liver injury).
- Shah RR. Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Saf. 2019;42:235–45. [PubMed: 30649740](Review of the safety of histone deacetylase inhibitors approved for use in the US mentions that elevations in serum aminotransferase levels have been reported during therapy with romidepsin, panobinostat and belinostat but not with vorinostat, and there have been no reports of clinically apparent hepatotoxicity, except for a single case of hepatic failure arising during a clinical trial of belinostat).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Romidepsin.[LiverTox: Clinical and Researc...]Review Romidepsin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Panobinostat.[LiverTox: Clinical and Researc...]Review Panobinostat.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Phase I and pharmacokinetic study of the oral histone deacetylase inhibitor vorinostat in Japanese patients with relapsed or refractory cutaneous T-cell lymphoma.[J Dermatol. 2012]Phase I and pharmacokinetic study of the oral histone deacetylase inhibitor vorinostat in Japanese patients with relapsed or refractory cutaneous T-cell lymphoma.Wada H, Tsuboi R, Kato Y, Sugaya M, Tobinai K, Hamada T, Shimamoto T, Noguchi K, Iwatsuki K. J Dermatol. 2012 Oct; 39(10):823-8. Epub 2012 Apr 16.
- Review Belinostat.[LiverTox: Clinical and Researc...]Review Belinostat.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents.[J Cancer Res Clin Oncol. 2016]Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents.Xue K, Gu JJ, Zhang Q, Mavis C, Hernandez-Ilizaliturri FJ, Czuczman MS, Guo Y. J Cancer Res Clin Oncol. 2016 Feb; 142(2):379-87. Epub 2015 Aug 28.
- Vorinostat - LiverToxVorinostat - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...