NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Vitamin E (alpha tocopherol) is a fat soluble vitamin and potent antioxidant that is believed to be important in protecting cells from oxidative stress, regulating immune function, maintaining endothelial cell integrity and balancing normal coagulation. There is no evidence that vitamin E, in physiologic or even super-physiologic, high doses, causes liver injury or jaundice.
Background
Vitamin E is a fat soluble vitamin known chemically as alpha-tocopherol (toe kof' er ol). Vitamin E is a potent antioxidant and is believed to be important is protecting cells from oxidative stress. Alpha tocopherol exists in 8 isomeric forms, but only one (R-R-R alpha tocopherol, “natural” vitamin E) has full antioxidant activity and is the predominant form found in nature in foods rich in vitamin E. Vitamin E is taken up my multiple cells and becomes embedded in the endoplasmic reticulum where it carries out antioxidant activity, maintaining the integrity of intracellular pathways and molecules than can be altered or inactivated by reactive oxygen radicals. Deficiency of vitamin E is rare, found largely in patients with severe fat malabsorption, with advanced liver disease or on total parenteral nutrition. Vitamin E is available in multiple forms, including tablets, capsules, oral solutions and syrups and solutions for intravenous administration (for parenteral nutrition). Most formulations are available over the counter, but many are mixed isomers of alpha tocopherol and the biologic activity of these products is unclear. The recommended daily allowance (RDA) for vitamin E is 15 mg (22 IU; 35 µmol) daily in adults and varies in children from 4 mg in neonates ages 0 to 6 months, 5 mg in infants ages 7 to 12 months, 6 mg for ages 1 to 3 years, 7 mg for ages 4 to 8 years, 11 mg for ages 9 to 13 years, to 15 mg for adolescents ages 14 years and above. Recent surveys suggest that the average American diet provides less than the RDA levels for vitamin E. An adequate blood level of vitamin E is considered 4 mg/mL. Vitamin E has been evaluated as therapy or means of prevention of many diseases including coronary artery disease, stroke, cognitive decline, macular degeneration and multiple forms of cancer, but has not been shown to have a significant effect on the incidence, morbidity or mortality of these diseases. Recently, small trials have suggested that vitamin E supplements can decrease steatosis, inflammation and cell injury in patients with nonalcoholic fatty liver disease. In doses below 1000 mg (1,500 IU; 2325 µmol) daily, vitamin E has been found to be safe and without adverse events. Patients on anticoagulant therapy may have an increase in bleeding episodes because of vitamin E’s effect on platelet aggregation.
Hepatotoxicity
Neither normal nor excessively high intakes of vitamin E are associated with liver injury or liver test abnormalities. In long term clinical trials, serum enzyme and bilirubin elevations were no more frequent with vitamin E therapy than with placebo. Indeed, in many animal models, vitamin E is protective against hepatotoxic substances and provides antioxidant and cytoprotective activity to hepatocytes. In several randomized controlled trials, vitamin E has been found to improve serum aminotransferase elevations and liver biopsy histologic findings of steatosis, inflammation and cell injury in patients with nonalcoholic steatohepatitis (NASH). Therapy was well tolerated and no patients had significant rises in serum aminotransferase levels during treatment or worsening of the underlying liver injury. While liver histology improved including steatosis, cell injury and inflammation, therapy was not associated with improvements in liver fibrosis and the role of vitamin E in the long term management of nonalcoholic fatty liver disease is not clear. However, in multiple controlled trials of vitamin E therapy there have been no reports of significant liver injury or jaundice with treatment.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
It is not clear how vitamin E might cause liver injury. Vitamin E is taken up and metabolized by multiple tissues and does not undergo metabolism by the liver cytochrome P450 system.
Drug Class: Vitamins
Other Drugs in the Class: Vitamin A, Vitamin B, Vitamin C, Vitamin D, Vitamin K, Folate, Niacin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Vitamin E – Generic
DRUG CLASS
Vitamins
Product labeling at DailyMed, National Library of Medicine, NIH
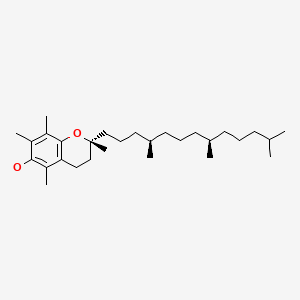
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| alpha-Tocopherol | 59-02-9 | C29-H50-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 28 May 2021
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999; does not discuss vitamin E).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp, 631-57.(Review of hepatotoxicity of dietary supplements; does not discuss vitamins and minerals).
- Traber MG. Vitamin E. In, Shils ME, Olson JA, Shike M, Ross AC, eds. Modern nutrition in health and disease. 9th ed. Baltimore: Williams & Wilkins, 1998; pp 347-62.(Textbook of nutrition).
- Food and Nutrition Board, Institute of Medicine. DRI dietary reference intakes: for vitamin C, vitamin E, selenium and carotenoids. Washington DC: National Academy Press, 1998.(Reports from the Food and Nutrition Board of the Institute of Medicine on dietary reference values for vitamin E intake, replacing the previously published Recommended Dietary Allowances).
- Office of Dietary Supplements. https://ods
.od.nih.gov /factsheets/VitaminE-HealthProfessional/ (Fact sheet on vitamin E maintained and regularly updated by the Office of Dietary Supplements, National Institutes of Health). - Diplock AT. Safety of antioxidant vitamins and beta-carotene. Am J Clin Nutr. 1995;62(6) Suppl:1510S–1516S. [PubMed: 7495252](Review of animal and human studies of safety of vitamin E uncovered no apparent toxicity; “A dosage of ≤1000 mg/d is considered to be entirely safe and without side effects”).
- Meyers DG, Maloley PA, Weeks D. Safety of antioxidant vitamins. Arch Intern Med. 1996;156:925–35. [PubMed: 8624173](Review of adverse effects of vitamin E mentions that several studies have shown the safety of vitamin E, but individual reports have mentioned a variety of side effects such as thrombophlebitis, headache, dizziness, urticarial and hypertension; liver toxicity in neonates was noted with an intravenous preparation that has subsequently been withdrawn and the complication has not been seen since).
- Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–45. [PubMed: 15817846](Review of the safety of vitamin E: “More than 20 published clinical trials involving ≥80,000 subjects have documented the safety of vitamin E supplements”).
- Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. [PubMed: 15998891](Results of the Women’s Health Study of vitamin E [600 IU] vs placebo every other day for ten years in 39,876 healthy women [ages ≥45 years] found no decrease in overall mortality, stroke, cardiovascular events or cancer with vitamin E therapy, and no differences in rates of side effects except for slight increase in risk for nose bleeds).
- Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. [PMC free article: PMC2586922] [PubMed: 18997197](Results of the Physician’s Health Study comparing therapy with vitamin E [400 IU every other day] with vitamin C [500 mg daily] versus placebo for an average of 8 years, found no effect of vitamin E on cardiovascular events or mortality, although vitamin E was associated with increase in hemorrhage stroke).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to vitamin E).
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, et al. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. [PMC free article: PMC2928471] [PubMed: 20427778](Among 247 adults with NASH treated with pioglitazone, vitamin E [800 IU daily] or placebo for 2 years, vitamin E therapy was associated with higher rates of biochemical and histological improvements than placebo, and side effects were similar).
- Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68. [PMC free article: PMC3110082] [PubMed: 21521847](Among 173 children with NASH treated with vitamin E, metformin or placebo for 2 years, vitamin E was associated with a higher rate of histological resolution of NASH and marginal higher rates of improvements in ALT levels compared to placebo; side effects were similar).
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. American Association for the Study of Liver Diseases. American College of Gastroenterology; American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–26. [PubMed: 22641309](Guidelines from management of nonalcoholic fatty liver disease based upon expert opinion from 3 academic societies, recommends use of vitamin E [800 IU daily] for non-diabetic adult patients with biopsy proven NASH without cirrhosis).
- Fortmann SP, Burda BU, Senger CA, Lin JS, Beil TL, O'Connor E, Whitlock EP. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: a systematic evidence review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Available from http://www
.ncbi.nlm.nih .gov/books/NBK173987/. [PubMed: 24308073] (Systematic review of efficacy and safety of vitamin E in prevention of cardiovascular disease and cancer in 6 large controlled trials involving 120,355 patients found associations in several studies, but none that were consistently reported). - Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, et al. Non-alcoholic Steatohepatitis Clinical Research Network. (NASH CRN). Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–43. [PMC free article: PMC3775262] [PubMed: 23718573](In a randomized controlled trial therapy with vitamin E (800 IU daily) was more effective than placebo in improving serum ALT levels [48% vs 12%] and improving liver steatosis, necrosis and inflammation, but not fibrosis and these effects were independent of weight loss).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 7 were attributed to niacin, but none were attributed to any other vitamin including vitamin E).
- Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–90. [PubMed: 27646933](Review of the many agents being evaluated as therapy for nonalcoholic steatohepatitis, their evidence for efficacy, safety and mechanisms of action; vitamin E is considered an antioxidant and has few adverse effects, but long term, higher dose therapy has been variably implicated in increased risk of cardiovascular disease, stroke and cancer; no mention of hepatotoxicity).
- Miyashima Y, Shibata M, Honma Y, Matsuoka H, Hiura M, Abe S, Harada M. Severe alcoholic hepatitis effectively treated with vitamin E as an add-on to corticosteroids. Intern Med. 2017;56:3293–7. [PMC free article: PMC5790716] [PubMed: 29021453](49 year old woman with severe acute alcoholic hepatitis failed to improve rapidly with high dose corticosteroid therapy, but improved promptly upon addition of oral vitamin E [150-300 mg daily]).
- Bordbar M, Shakibazad N, Fattahi M, Haghpanah S, Honar N. Effect of ursodeoxycholic acid and vitamin E in the prevention of liver injury from methotrexate in pediatric leukemia. Turk J Gastroenterol. 2018;29:203–9. [PMC free article: PMC6284693] [PubMed: 29749328](Among 80 children with acute myelogenous leukemia being treated with methotrexate, mercaptopurine and corticosteroids who were given supplemental vitamin E, ursodiol, or both or neither, rates of liver injury were similar in all groups which led them to conclude that they “failed to prove the true benefits of supplements”).
- Podszun MC, Alawad AS, Lingala S, Morris N, Huang WA, Yang S, Schoenfeld M, et al. Vitamin E treatment in NAFLD patients demonstrates that oxidative stress drives steatosis through upregulation of de-novo lipogenesis. Redox Biol. 2020;37:101710. [PMC free article: PMC7494510] [PubMed: 32920226](Analysis of liver histology, magnetic resonance spectroscopy and serum markers in 20 patients with nonalcoholic steatohepatitis treated with variable doses of vitamin E found evidence of decreased in hepatic triglyceride accumulation and de novo lipogenesis with vitamin E therapy, which was associated with improvements in histologic features of steatosis, inflammation and ballooning degeneration).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat.[Poult Sci. 2011]Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat.Voljc M, Frankic T, Levart A, Nemec M, Salobir J. Poult Sci. 2011 Jul; 90(7):1478-88.
- Vitamin E analogs limit in vitro oxidant damage to bovine mammary endothelial cells.[J Dairy Sci. 2021]Vitamin E analogs limit in vitro oxidant damage to bovine mammary endothelial cells.Kuhn MJ, Sordillo LM. J Dairy Sci. 2021 Jun; 104(6):7154-7167. Epub 2021 Mar 25.
- Review Vitamin K.[LiverTox: Clinical and Researc...]Review Vitamin K.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Effects of oral micellized natural vitamin E (D-α-tocopherol) v. synthetic vitamin E (DL-α-tocopherol) in feed on α-tocopherol levels, stereoisomer distribution, oxidative stress and the immune response in piglets.[Animal. 2014]Effects of oral micellized natural vitamin E (D-α-tocopherol) v. synthetic vitamin E (DL-α-tocopherol) in feed on α-tocopherol levels, stereoisomer distribution, oxidative stress and the immune response in piglets.Amazan D, Cordero G, López-Bote CJ, Lauridsen C, Rey AI. Animal. 2014 Mar; 8(3):410-9.
- Review Cooperation of liver cells in health and disease.[Adv Anat Embryol Cell Biol. 2001]Review Cooperation of liver cells in health and disease.Kmieć Z. Adv Anat Embryol Cell Biol. 2001; 161:III-XIII, 1-151.
- Vitamin E - LiverToxVitamin E - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...