NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Venetoclax is an oral selective BCL-2 inhibitor and antineoplastic agent used in the therapy of refractory chronic lymphocytic leukemia (CLL). Venetoclax is associated with a low rate of transient serum enzyme elevations during therapy, but has not been implicated in cases of clinically apparent acute liver injury with jaundice. Venetoclax has potent immunosuppressive activity and may be capable of causing reactivation of hepatitis B.
Background
Venetoclax (ven et' oh klax) is a small molecule inhibitor of BCL-2, an intracellular protein that inhibits apoptosis. BCL2 is overexpressed in cancer cells, particularly in chronic lymphocyte leukemia (CLL). Overexpression of BCL2 increases cancer cell survival and increases resistance to chemotherapy. Venetoclax binds directly to BCL2 and blocks its antiapoptotic activity, leading to programmed cell death in the malignant B cells. Venetoclax was found to have activity against refractory and relapsed CLL and was approved for use as an antineoplastic agent in the United States in 2016. Current indications are limited to therapy of previously treated patients with CLL and an accompanying 17p chromosomal deletion (which is associated with a poor prognosis and resistance to standard chemotherapy). Venetoclax is available as tablets of 10, 50 and 100 mg under the brand name Venclexta. The typical initial adult dose for CLL 20 mg once daily for one week with gradual dose escalation (“ramp-up”) to as much as 400 mg daily. Side effects are common and include bone marrow suppression, neutropenia, infections, fever, diarrhea, nausea and vomiting, anorexia, abdominal discomfort and fatigue. Severe side effects include pneumonia, neutropenic fever and sepsis, autoimmune hemolytic anemia, embryo-fetal toxicity and tumor lysis syndrome (for which prophylactic measures are recommended during the ramp-up period).
Hepatotoxicity
In clinical trials in 240 patients with CLL, serum aminotransferase elevations occurred in 20% of subjects treated with venetoclax, but the elevations were generally transient, mild and not associated with jaundice or symptoms. In the preregistration trials, no cases of clinically apparent liver injury attributed to venetoclax were reported and few patients required drug discontinuation for liver test abnormalities. Since approval, venetoclax has had limited clinical use, but has not been implicated in cases of clinically apparent liver injury. Venetoclax decreases total white blood cell counts and can cause lymphopenia in addition to neutropenia. As a consequence, venetoclax may be capable of inducing immune reactions including reactivation of hepatitis B. However, instances of reactivation have not been reported, but neither has detailed information on the effects of venetoclax on hepatitis B virus levels in patients with preexisting hepatitis B or evidence of previous infection.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Venetoclax is unlikely to have direct hepatotoxic effects as dose escalation studies showed no dose related effect on liver test results. On the other hand, the immunosuppressive effects of venetoclax may cause liver related changes that could lead to viral or autoimmune reactions. Venetoclax is metabolized in the liver, largely via CYP 3A4 and is susceptible to drug-drug interactions with inhibitors or inducers of CYP 3A or in inhibitors or substrates of P-glycoprotein.
Outcome and Management
Patients receiving venetoclax require careful monitoring largely for hematologic abnormalities and blood chemistry changes of tumor lysis syndrome. If serum aminotransferase levels rise to 5 times the upper limit of normal (ULN), therapy should be interrupted until levels fall to baseline or less than 1.5 times ULN.
Drug Class: Antineoplastic Agents, Antimetabolites
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Venetoclax – Venclexta®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Venetoclax | 1257044-40-8 | C45-H50-Cl-N7-O7-S |
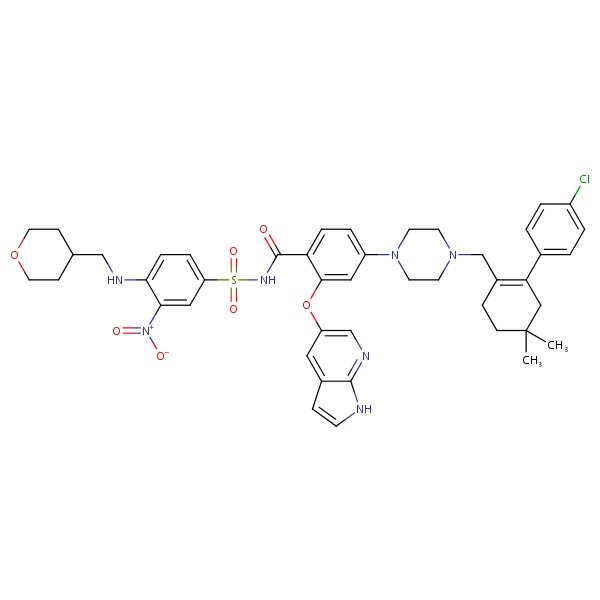 |
ANNOTATED BIBLIOGRAPHY
References updated: 04 April 2017
Abbreviations used: CLL, chronic lymphocytic leukemia; AML, acute myelogenous leukemia
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; no mention of venetoclax).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of hepatotoxicity of anticancer agents; does not discuss venetoclax).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan DP. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-53.(Textbook of pharmacology and therapeutics).
- Khaw SL, Mérino D, Anderson MA, Glaser SP, Bouillet P, Roberts AW, Huang DC. Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective pharmacological inhibition of prosurvival Bcl-2 with ABT-199. Leukemia 2014; 28: 1207-15. [PubMed: 24402163](While CLL cells are highly sensitive to venetoclax, so are normal peripheral blood B cells, although not T cells, myeloid cells, thrombocytes or B-cell precursors).
- Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016; 374: 311-22. [PMC free article: PMC7107002] [PubMed: 26639348](Among 116 adults with CLL treated with varying doses of venetoclax, the overall response rate was 79% with complete remissions in 20%, while adverse events occurred in 99% and were serious in 83% of patients, including tumor lysis syndrome [one fatality], diarrhea, nausea, neutropenia, fatigue and fever; no mention of ALT elevations or hepatotoxicity).
- Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, Puvvada SD, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 2016; 17: 768-78. [PubMed: 27178240](Among 107 adults with relapsed or refractory CLL [with the 17p deletion] treated with venetoclax in a dose ramp-up schedule to 400 mg daily for a median of 12 months, the overall response rate was 79% while serious adverse events occurred in 55% including neutropenia [40%], autoimmune hemolytic anemia [7%], pneumonia [7%], febrile neutropenia [5%] and no patient developed “abnormal hepatic function” except 1 who died of “liver derangement” that was considered not related to treatment).
- Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 2016; 6: 1106-17. [PMC free article: PMC5436271] [PubMed: 27520294](Among 32 patients with relapsed or refractory AML treated with venetoclax, the overall response rate was 19% and adverse events occurred in all patients and were serious in 84%; no mention of ALT elevations or hepatotoxicity).
- Deeks ED. Venetoclax: First global approval. Drugs 2016; 76: 979-87. [PubMed: 27260335](Summary of the pharmacology, clinical efficacy and safety of venetoclax).
- Venetoclax (Venclexta) for chronic lymphocytic leukemia. Med Lett Drugs Ther 2016; 58 (1500): 101-2. [PubMed: 27466751](Concise review of the mechanism of action, clinical efficacy, safety and costs of venetoclax shortly after its approval in the US; discusses its major side effects, but does not mention ALT elevations or hepatoxicity).
- Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, Anderson MA, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 2017; 18: 230-40. [PMC free article: PMC5316338] [PubMed: 28089635](Among 49 adults with relapsed or refractory CLL treated with venetoclax and monthly rituximab, the overall response rate was 86% with a complete response in 51%; adverse events were common including diarrhea, fatigue, neutropenia and infection, while ALT elevations occurred in only 2 patients which were above 5 times ULN in one).
- King AC, Peterson TJ, Horvat TZ, Rodriguez M, Tang LA. Venetoclax: A first-in-class oral BCL-2 inhibitor for the management of lymphoid malignancies. Ann Pharmacother 2017; 51: 410-6. [PubMed: 28056525](Review of the pharmacology, clinical efficacy safety and costs of venetoclax; discusses tumor lysis syndrome and its prevention, but does not mention ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Fludarabine.[LiverTox: Clinical and Researc...]Review Fludarabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review BCL-2 Inhibition as Treatment for Chronic Lymphocytic Leukemia.[Curr Treat Options Oncol. 2021]Review BCL-2 Inhibition as Treatment for Chronic Lymphocytic Leukemia.Perini GF, Feres CCP, Teixeira LLC, Hamerschlak N. Curr Treat Options Oncol. 2021 Jun 10; 22(8):66. Epub 2021 Jun 10.
- Phase 1/2 study of venetoclax, a BCL-2 inhibitor, in Japanese patients with relapsed or refractory chronic lymphocytic leukemia and small lymphocytic lymphoma.[Int J Hematol. 2021]Phase 1/2 study of venetoclax, a BCL-2 inhibitor, in Japanese patients with relapsed or refractory chronic lymphocytic leukemia and small lymphocytic lymphoma.Izutsu K, Yamamoto K, Kato K, Ishikawa T, Fukuhara N, Terui Y, Choi I, Humphrey K, Kim SY, Okubo S, et al. Int J Hematol. 2021 Mar; 113(3):370-380. Epub 2020 Oct 23.
- Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax.[Int J Mol Sci. 2023]Landscape of BCL2 Resistance Mutations in a Real-World Cohort of Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia Treated with Venetoclax.Kotmayer L, László T, Mikala G, Kiss R, Lévay L, Hegyi LL, Gróf S, Nagy T, Barna G, Farkas P, et al. Int J Mol Sci. 2023 Mar 18; 24(6). Epub 2023 Mar 18.
- Review Venetoclax in the Treatment of Chronic Lymphocytic Leukemia: Evidence, Expectations, and Future Prospects.[Cureus. 2020]Review Venetoclax in the Treatment of Chronic Lymphocytic Leukemia: Evidence, Expectations, and Future Prospects.Tariq S, Tariq S, Khan M, Azhar A, Baig M. Cureus. 2020 Jun 29; 12(6):e8908. Epub 2020 Jun 29.
- Venetoclax - LiverToxVenetoclax - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...