NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Trifluoperazine is a phenothiazine and antipsychotic agent that no longer commonly used in clinical practice. Trifluoperazine is a rare cause of clinically apparent acute cholestatic liver injury.
Background
Trifluoperazine (trye" floo oh per' a zeen) is a piperazine phenothiazine derivative which acts by postsynaptic inhibition of dopamine receptors. Trifluoperazine has other peripheral and central nervous system effects, producing both alpha adrenergic stimulation and blocking histamine- and serotonin-mediated effects. Trifluoperazine is indicated for the therapy of acute and chronic psychosis and rarely for nonpsychotic anxiety. Trifluoperazine was approved for use in the United States in 1959 and was formerly a commonly prescribed antipsychotic medication, but has been replaced in recent years in large part by the atypical antipsychotics, which have fewer extrapyramidal side effects. Trifluoperazine is available as tablets of 1, 2, 5 and 10 mg in generic forms and formerly under the brand name of Stelazine. Oral solutions and parenteral formulations are also available. The typical recommended dose for nonpsychotic anxiety in adults in 1 to 2 mg twice daily, increasing as needed to a maximum of 6 mg daily. For therapy of schizophrenia the typical dose is 2 to 5 mg twice daily increasing as need to a maximum of 15 to 40 mg per day. Common side effects include drowsiness, dizziness, headache, blurred vision, dry mouth, constipation, tremor, restlessness, muscle spasms and weight gain. Uncommon but potentially severe adverse events include increased mortality in elderly patients with dementia-associated psychosis, neuroleptic malignant syndrome, tardive dyskinesia, hypotension and falls.
Hepatotoxicity
Liver test abnormalities have been reported to occur in a high proportion of patients on long term phenothiazine therapy, but elevations are uncommonly above 3 times the upper limit of normal. The aminotransferase abnormalities are usually mild, asymptomatic and transient, reversing even with continuation of medication. Rare instances of clinically apparent acute liver injury, resembling that due to chlorpromazine, have been reported with trifluoperazine therapy. The onset of jaundice is usually within 1 to 4 weeks, and the pattern of serum enzyme elevations is typically cholestatic or mixed. Immunoallergic features (rash, fever and eosinophilia) were present in some cases but were mild and self-limited; autoantibodies were rare.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which phenothiazines cause serum aminotransferase elevations is not known. Several features of the clinical presentation of phenothiazine hepatotoxicity (short latency period, fever, eosinophilia) suggest a hypersensitivity reaction, and rechallenge typically causes a rapid recurrence of injury. Trifluoperazine is extensively metabolized by the liver via sulfoxidation and oxidation, and some instances of serum aminotransferase elevations as well as more clinical apparent liver injury may be caused by production of a toxic intermediate of its metabolism.
Outcome and Management
The serum aminotransferase elevations that occur on trifluoperazine therapy are usually transient and do not require dose modification or discontinuation of therapy. The acute cholestatic hepatitis caused by trifluoperazine is typically self-limited and benign. However, a proportion of cases of phenothiazine cholestatic hepatitis may be followed by prolonged jaundice and cholestasis and vanishing bile duct syndrome. Many patients with chronic cholestasis eventually improve, but they often have persistent enzyme elevations and biliary cirrhosis. No fatalities from trifluoperazine jaundice have been reported. Rechallenge with phenothiazines usually causes a prompt recurrence of the liver injury and should be avoided. Patients with trifluoperazine induced liver injury may have cross sensitivity to other phenothiazines, but generally tolerate haloperidol and the atypical antipsychotics.
Drug Class: Antipsychotic Agents
Other Drugs in the Subclass, Phenothiazines: Chlorpromazine, Fluphenazine, Perphenazine, Prochlorperazine, Thioridazine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Trifluoperazine – Generic, Stelazine®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
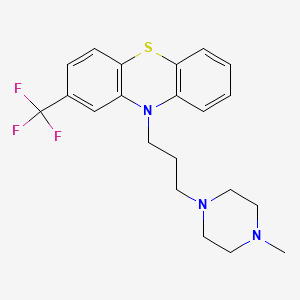
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Trifluoperazine | 117-89-5 | C21-H24-F3-N3-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 01 July 2020
- Zimmerman HJ. Neuroleptic drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-91.(Expert review of hepatotoxicity of neuroleptic drugs including the phenothiazines published in 1999; several hundred cases of chlorpromazine jaundice have been reported due to chlorpromazine, usually cholestatic, arising after 1-5 weeks, often with fever and eosinophilia, sometimes causing vanishing bile duct syndrome; other phenothiazines have only rarely been linked to liver injury, except for prochlorperazine).
- Larrey D, Ripault M-P. Phenothiazines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 452.(Review of phenothiazine hepatotoxicity mentions that liver enzyme elevations arise in up to 40% of patients taking phenothiazines and hundreds of cases of chlorpromazine jaundice have been published, frequency ~0.5-1%; onset in 2-5 weeks, usually acute cholestatic hepatitis with jaundice and pruritus; prodrome of fever and abdominal pain is common; prolonged course in 7% but usually benign; other phenothiazines have been linked to liver injury similar to that of chlorpromazine, “but with a lower frequency”).
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Kohn N, Myerson RM. Cholestatic hepatitis associated with trifluoperazine. N Engl J Med. 1961;264:549–50. [PubMed: 13757600](48 year old man developed jaundice and abdominal pain 3 weeks after starting trifluoperazine [bilirubin 5.2 mg/dL, AST 95 U/L, Alk P ~10 x ULN, eosinophils 6%], resolving within 4 weeks of stopping).
- McQueen EG. Toxic effects of phenothiazine tranquillizers. N Z Med J. 1963;62:460–2. [PubMed: 14073060](Review of the phenothiazines and their side effects: “Jaundice has occurred in about 1% of patients taking chlorpromazine, and also, although less frequently, in patients taking one of the more recently developed analogues”).
- Walker CO, Combes B. Biliary cirrhosis induced by chlorpromazine. Gastroenterology. 1966;51:631–40. [PubMed: 5926937](32 year old woman and 31 year old man developed persistent jaundice [>4 years], cholestasis and liver fibrosis 3 and 4 weeks after starting chlorpromazine; acute cholestatic hepatitis evolving into chronic form with biopsies showing cirrhosis and complications of portal hypertension, most likely representing vanishing bile duct syndrome).
- Margulies AI, Berris B. Jaundice associated with the administration of trifluoperazine. Can Med Assoc J. 1968;98:1063–4. [PMC free article: PMC1924193] [PubMed: 5652451](26 year old woman developed fever, rash and pruritus 2 weeks after starting trifluoperazine [bilirubin 6.4 mg/dL, ALT 169 U/L, Alk P ~1.8 times normal, 8% eosinophils], resolving within 1 month stopping and later tolerating haloperidol without recurrence).
- Ishak KG, Irey NS. Hepatic injury associated with the phenothiazines. Clinicopathologic and follow-up study of 36 patients. Arch Pathol. 1972;93:283–304. [PubMed: 5017281](Review of 36 biopsies from patients with phenothiazine induced liver injury from the files of the Armed Forces Institute of Pathology, 33 chlorpromazine, 3 prochlorperazine; mean onset 15 days after starting; eosinophilia in 73%, mean bilirubin 12.4 mg/dL, Alk P ~8 fold elevated, ALT 146 U/L; 6 [17%] had prolonged course for 10-16 months).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol. 1982;17:205–11. [PubMed: 6982502](Among 572 cases of drug induced liver disease seen between 1968-78 in Denmark, 51 were attributed to chlorpromazine [9%, ranking 2nd behind halothane], latency averaging 14 days [range 11-46]; 5 deaths; no other phenothiazines mentioned).
- Kaplowitz N, Aw TY, Simon FR, Stolz A. Drug-induced hepatotoxicity. Ann Intern Med. 1986;104:826–39. [PubMed: 3518564](Review of drug induced hepatotoxicity including phenothiazine jaundice).
- Munyon WH, Salo R, Briones DF. Cytotoxic effects of neuroleptic drugs. Psychopharmacology (Berl). 1987;91:182–8. [PubMed: 2883697](In vitro cell cytotoxicity of 8 neuroleptic drugs were compared; chlorpromazine was more toxic than haloperidol or loxapine, but similar to other phenothiazines).
- Regal RE, Bili JE, Glazer HM. Phenothiazine-induced cholestatic jaundice. Clinical Pharmacy. 1987;6:787–94. [PubMed: 2905941](Review of phenothiazine induced liver injury; cross sensitivity is rare “but does exist”).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years experience. N Z Med J. 1996;109:315–9. [PubMed: 8816722](Over 21 year period in New Zealand, there were 943 reports of liver injury involving 205 drugs; chlorpromazine was in the top 20 drugs implicated accounting for 2.7% of cases; prochlorperazine was cause of 4 cases, but other phenothiazines not mentioned).
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. [PubMed: 10553730](Systematic review of 81 articles on weight change with antipsychotics; using change after 10 weeks to compare agents: clozapine +5.7, olanzapine +4.2, chlorpromazine +4.2, risperidone +1.7, loxapine +0.6, haloperidol +0.5, ziprasidone +0.3, molindone -0.1, and pimozide -2.7 kilograms).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993 and 2000, 3 were due to chlorpromazine with relative risk of 613: frequency of 261 per 100,000 person year exposures; other phenothiazines not mentioned).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were due to phenothiazines).
- Flanagan RJ. Fatal toxicity of drugs used in psychiatry. Hum Psychopharmacol. 2008;23 Suppl 1:43–51. [PubMed: 18098225](Deaths from fatal poisonings decreased in England and Wales between 1993-2004, antipsychotic overdose fatalities higher for phenothiazines than atypicals; deaths/million prescriptions being 29 for chlorpromazine, 15.5 thioridazine, 3.9 trifluoperazine, 13.3 olanzapine, 21 clozapine and 31.3 quetiapine).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 4 due to psychotropic agents; one each for quetiapine, nefazodone, fluoxetine and venlafaxine, but none for phenothiazines).
- Sharifi V, Amini S, Ghaeli P, Saeedinia A. Trifluoperazine-induced cholestatic jaundice. Iran J Psychiatry. 2010;5:117–8. [PMC free article: PMC3430503] [PubMed: 22952504](44 year old man developed jaundice 4 weeks after starting trifluoperazine [bilirubin 3.9 mg/dL, ALT 235 U/L, Alk P 744 U/L], resolving with stopping and not recurring after switching to risperidone).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with suspected drug induced liver injury, half [n=15] were due to antimicrobials [minocycline 4, INH 3, azithromycin 3] and the rest largely due to CNS agents and anticonvulsants; one case was attributed to perphenazine).
- Drugs for psychiatric disorders. Treat Guidel Med Lett. 2013;11(130):53–64. [PubMed: 23715100](Concise review and recommendations on use of antidepressants and antipsychotic medications including phenothiazines; no discussion of hepatotoxicity).
- Marwick KF, Taylor M, Walker SW. Antipsychotics and abnormal liver function tests: systematic review. Clin Neuropharmacol. 2012;35:244–53. [PubMed: 22986798](Systematic review of the literature found rates of any serum enzyme elevation during antipsychotic therapy to range from 5-78% and "clinically significant' elevations in 0-15%; lists 3 reports of clinically apparent liver injury due trifluoperazine]).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, only one of which was attributed to chlorpromazine, the only antipsychotic medication listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 cases [0.6%] were attributed to antipsychotic agents, including 3 due to quetiapine and 2 to olanzapine, but none to trifluoperazine or other phenothiazines]).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that the phenothiazines commonly cause sedation, postural hypotension and weight gain and often have extrapyramidal effects, but does not mention ALT elevations or hepatotoxicity).
- Schreiner NM, Windham S, Barker A. Atypical neuroleptic malignant syndrome: diagnosis and proposal for an expanded treatment algorithm: a case report. A A Case Rep. 2017;9:339–43. [PubMed: 28767476](48 year old man with bipolar disorder and NASH underwent liver transplantation and developed confusion, dyskinesia, rigidity, hyperthermia and tachycardia/tachypnea postoperatively having been given lithium, lamotrigine, promethazine and ziprasidone, responding to therapy of neuroleptic malignant syndrome with benzodiazepines and propofol).
- Baeza I, de la Serna E, Calvo-Escalona R, Merchán-Naranjo J, Rodríguez-Latorre P, Martínez-Cantarero MC, Andrés P, et al. One-year prospective study of liver function tests in children and adolescents on second-generation antipsychotics: is there a link with metabolic syndrome? J Child Adolesc Psychopharmacol. 2018;28:463–73. [PubMed: 29975563](Among 216 children and adolescents starting atypical antipsychotics, mean weight gain at 6 months was 6.5 kg and mean ALT levels increased by 8.6 U/L, while among 37 taking olanzapine mean weight gain was 10.3 kg and ALT increase 2.6 U/L; increases in ALT associated with development of the metabolic syndrome, mean ALT increasing by 27.8 U/L at 6 months).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Perphenazine.[LiverTox: Clinical and Researc...]Review Perphenazine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Thioridazine.[LiverTox: Clinical and Researc...]Review Thioridazine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fluphenazine.[LiverTox: Clinical and Researc...]Review Fluphenazine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer.[Am J Respir Crit Care Med. 2012]Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer.Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, Ho CC, Chen CC, Kuo YL, Lee PY, et al. Am J Respir Crit Care Med. 2012 Dec 1; 186(11):1180-8. Epub 2012 Sep 28.
- Review Chlorpromazine.[LiverTox: Clinical and Researc...]Review Chlorpromazine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Trifluoperazine - LiverToxTrifluoperazine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...