NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tizanidine is a commonly used muscle relaxant that has been linked to rare instances of acute liver injury, a few of which have been fatal.
Background
Tizanidine (tye zan' i deen) is an imidazoline derivative and is a centrally acting muscle relaxant used for therapy of acute muscle spasms and chronic spasticity. The mechanism by which tizanidine causes skeletal muscle relaxation is not well known; it appears to act at the level of spinal cord pain reflexes, most likely through activity as a central alpha-adrenergic agonist which results in an decrease in activity of motor neurons. Tizanidine was approved for use in the United States in 1996 and currently several million prescriptions are filled yearly. The current indications are limited to short-term management of spasticity. Tizanidine is available in several generic forms as well as under the brand name of Zanaflex in tablets and capsules of 2, 4 or 6 mg. The recommended dose in adults is 2 to 6 mg orally three to four times daily. Common side effects include tiredness, drowsiness, dizziness, muscular weakness, dry mouth and occasionally hypotension.
Hepatotoxicity
Transient and asymptomatic elevations in serum ALT greater than 3 times the upper limit of normal occur in ~5% of patients taking tizanidine compared to 0.4% of subjects on placebo. Reports of severe hepatotoxicity, acute liver failure and death have been mentioned in review articles on tizanidine, but few case reports have been published. In these reports, the latency to onset of jaundice has ranged from 2 to 14 weeks and the enzyme elevations have been both cholestatic and hepatocellular (Case 1). Immunoallergic and autoimmune features have not been mentioned. Recovery was complete after 1 to 2 months.
Likelihood score: C (Probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of acute hepatic injury from tizanidine is unknown, but is probably idiosyncratic due to hypersensitivity.
Outcome and Management
The idiosyncratic liver injury due to tizanidine ranges from hepatitis with jaundice to acute liver failure leading to death. Chronic injury and vanishing bile duct syndrome have not been reported after tizanidine therapy. Recurrence on re-exposure has been reported and rechallenge should be avoided. No cross reactivity with other muscle relaxants has been identified.
Drug Class: Muscle Relaxants
CASE REPORT
Case 1. Acute hepatocellular injury with jaundice after tizanidine therapy.
[Modified from: de Graaf EM, Oosterveld M, Tjabbes T, Stricker BH. A case of tizanidine-induced hepatic injury. J Hepatol 1996; 25: 772-3. PubMed Citation]
A 55 year old woman with multiple sclerosis and lower limb muscle spasms developed jaundice 4 months after starting tizanidine therapy. The jaundice had been preceded by a 2 week period of progressive fatigue, nausea and poor appetite. She denied a history of liver disease or exposure to hepatitis and did not drink alcohol. Her other medications included baclofen, chlormezanone, diazepam, flurazepam, dexchlorpheniramine and diclofenac, all of which she had been taking chronically. On examination, she was deeply jaundiced and somnolent. She had no signs of chronic liver disease and did not have rash or fever. Laboratory testing showed a total bilirubin of 26.5 mg/dL and marked elevations in serum aminotransferase levels with minimal increase in alkaline phosphatase (Table). Tests for hepatitis A, B and C were negative as were autoantibodies (smooth muscle antibody was marginally positive). An abdominal ultrasound showed no evidence of biliary obstruction. Tizanidine had been discontinued 2 weeks before admission, but she worsened during the next several weeks, but then began a spontaneous recovery which was complete 2 months later. Several months later, the patient was inadvertently rechallenged with a single 4 mg dose of tizanidine and within 6 days the ALT levels had increased from 14 to 155 U/L. Tizanidine was discontinued again, and one week later serum ALT levels had returned to normal.
Key Points
| Medication: | Tizanidine 36 mg daily |
| Pattern: | Hepatocellular (R=15) |
| Severity: | 4+ (jaundice, hospitalization, mild hepatic failure) |
| Latency: | 14 weeks to onset of symptoms |
| Recovery: | Complete recovery 2 months after stopping |
| Other medications: | Baclofen, diclofenac, chlormezanone, diazepam, flurazepam, dexchlorpheniramine |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Tizanidine started | ||||
| 2 days | 0 | Normal | Normal | Normal | |
| 16 days | 0 | Tizanidine stopped | |||
| 18 days | 2 days | 830 | 187 | 26.5 | Admission. INR=1.6 |
| 22 days | 8 days | 270 | 36.0 | ||
| 24 days | 10 days | 60 | 30.0 | ||
| 26 days | 12 days | 30 | 4.0 | ||
| 30 days | 16 days | 14 | 1.0 | Single dose of tizanidine | |
| 31 (1)** days | 17 (1) days | 155 | 1.0 | ||
| 32 (2) days | 18 (2) days | 30 | 1.0 | ||
| Normal Values | <30 | <100 | <1.2 | ||
* Bilirubin levels converted from μmol/L. Some values were estimated from Figure 1.
** Values in parentheses are the number of days after rechallenge
Comment
The onset of hepatic injury after 4 months of therapy, the improvement with stopping treatment, and the accelerated recurrence of hepatic injury with restarting tizanidine make it highly likely that the hepatitis was due to tizanidine induced liver injury. The pattern of injury was distinctly hepatocellular. Diclofenac can also cause similar hepatocellular injury, but the other features and particularly the rechallenge argue in support of tinazidine as the cause. The rapidity of recurrence on reexposure suggests that the hepatic injury was due to hypersensitivity. The muscle relaxants are a diverse group of medications and share little in structure or mechanisms of action, so that cross sensitivity to hepatic injury should not be a problem. This patient was restarted on baclofen and a benzodiazepam without complication.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tizanidine – Generic, Zanaflex®
DRUG CLASS
Autonomic Agents: Muscle Relaxants, Central
Product Labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tizanidine | 51322-75-9 | C9-H8-Cl-N5-S |
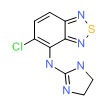 |
ANNOTATED BIBLIOGRAPHY
References updated: January 30, 2017
- Zimmerman HJ. Muscle spasmolytics. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 544-45.(Expert review of hepatotoxicity published in 1999; discusses dantrolene, chlorzoxazone and baclofen, but not tizanidine).
- Hibbs RE, Zambon AC. Agents acting at the neuromuscular junction and autonomic ganglia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011. p. 255-76. (Textbook of pharmacology and therapeutics).
- Fryda-Kaurimsky Z, Müller-Fassbender H. Tizanidine (DS 103-282) in the treatment of acute paravertebral muscle spasm: a controlled trial comparing tizanidine and diazepam. J Int Med Res 1981; 9: 501-5. [PubMed: 6459256](Among 10 patients treated with tizanidine, no mention of hepatic adverse effects).
- Smolenski C, Muff S, Smolenski-Kautz S. A double-blind comparative trial of new muscle relaxant, tizanidine (DS 103-282), and baclofen in the treatment of chronic spasticity in multiple sclerosis. Curr Med Res Opin 1981; 7: 374-83. [PubMed: 7016449](Among 21 patients treated with tizanidine or baclofen for 6 weeks, no mention of hepatotoxicity of either drug).
- Newman PM, Nogues M, Newman PK, Weightman D, Hudgson P. Tizanidine in the treatment of spasticity. Eur J Clin Pharmacol 1982; 23: 31-5. [PubMed: 6751834](Among 36 patients treated with tizanidine for chronic spasticity, 3 developed mild ALT elevations, but levels were below 50 U/L and resolved spontaneously).
- Lapierre Y, Bouchard S, Tansey C, Gendron D, Barkas WJ, Francis GS. Treatment of spasticity with tizanidine in multiple sclerosis. Can J Neurol Sci 1987; 14 (3 Suppl): 513-7. [PubMed: 3676923](Among 33 patients treated with tizanidine, 2 developed significant ALT and AST elevations, both resolved after stopping drug).
- Stien R, Nordal HJ, Oftedal SI, Slettebø M. The treatment of spasticity in multiple sclerosis: a double-blind clinical trial of a new anti-spastic drug tizanidine compared with baclofen. Acta Neurol Scand 1987; 75: 190-4. [PubMed: 3554879](Among 40 patients given either tizanidine or baclofen for 6 weeks, no changes observed in laboratory tests of liver function or mention made of hepatotoxicity of either drug).
- Bass B, Weinshenker B, Rice GP, Noseworthy JH, Cameron MGP, Hader W, Bouchard S, et al. Tizanidine versus baclofen in the treatment of spasticity in patients with multiple sclerosis. Can J Neurol Sci 1988; 15: 15-9. [PubMed: 3345456](Double blind cross over study of tizanidine vs baclofen for 5 weeks each; routine laboratory monitoring found no alterations in biochemical test results with either agent).
- Berry H, Hutchinson DR. A multicentre placebo-controlled study in general practice to evaluate the efficacy and safety of tizanidine in acute low-back pain. J Int Med Res 1988; 16: 75-82. [PubMed: 2967780](Among 112 patients in a placebo controlled trial [59 treated with tizanidine], no hepatic adverse events reported).
- Berry H, Hutchinson DR. Tizanidine and ibuprofen in acute low-back pain: results of a double-blind multicentre study in general practice. J Int Med Res 1988; 16: 83-91. [PubMed: 2967781](Placebo controlled trial of tizanidine with ibuprofen for 7 days in patients with low back pain; no hepatic adverse events reported in 51 tizanidine treated patients).
- Bes A, Eyssette M, Pierrot-Deseilligny E, Rohmer F, Warter JM. A multi-centre, double-blind trial of tizanidine, a new antispastic agent, in spasticity associated with hemiplegia. Curr Med Res Opin 1988; 10: 709-18. [PubMed: 3286129](Randomized controlled trial of tizanidine versus diazepam for 8 weeks in 105 patients with spasticity; there were no changes in “biochemical parameters” during the study).
- Eyssette M, Rohmer F, Serratrice G, Warter JM, Boisson D. Multi-centre, double-blind trial of a novel antispastic agent, tizanidine, in spasticity associated with multiple sclerosis. Curr Med Res Opin 1988; 10: 699-708. [PubMed: 3286128](Among 100 patients in a randomized controlled trial of baclofen vs tizanidine for spasticity, 1 on tizanidine developed bilirubin and ALT elevations and skin rash, but ALT levels had been elevated before therapy).
- Hoogstraten MC, van der Ploeg RJ, vd Burg W, Vreeling A, van Marle S, Minderhoud JM. Tizanidine versus baclofen in the treatment of spasticity in multiple sclerosis patients. Acta Neurol Scand 1988; 77: 224-30. [PubMed: 3376747](Among 16 patients treated with tizanidine and baclofen in a cross over study, muscle weakness was more common with baclofen; no mention of laboratory tests or hepatotoxicity).
- Lang AE, Riley DE. Tizanidine in cranial dystonia. Clin Neuropharmacol 1992; 15: 142-7. [PubMed: 1591739](Among 10 patients treated with tizanidine, one had elevated ALT levels before therapy which remained elevated during and after treatment).
- Wallace JD. Summary of combined clinical analysis of controlled clinical trials with tizanidine. Neurology 1994; 44 (Suppl 9): S60-8; discussion S68-9. [PubMed: 7970013](Review of 525 patients treated with tizanidine in 3 controlled trials, 2 patients developed significant liver enzyme elevations; but many had "slight" abnormalities [all <10 times ULN], which resolved with reduction of dosage or discontinuation).
- Rustemovic N, Huic M, Opacic M. Tinanidine-induced acute toxic hepatitis: case report. Pharmaca 1994; 32: 457-61. Not in PubMed.(Report of toxic hepatitis cited in Henney [2009]).
- de Graaf EM, Oosterveld M, Tjabbes T, Stricker BH. A case of tizanidine-induced hepatic injury. J Hepatol 1996; 25: 772-3. [PubMed: 8938559](55 year old woman developed jaundice 14 weeks after starting tizanidine [bilirubin 26.5 mg/dL, ALT 830 U/L, Alk P 187 U/L], resovling within 6 weeks; recurrence of ALT elevations after reexposure to a single tablet: Case 1).
- Groves L, Shellenberger MK, Davis CS. Tizanidine treatment of spasticity: a meta-analysis of controlled, double-blind, comparative studies with baclofen and diazepam. Adv Ther 1998; 15: 241-51. [PubMed: 10186943](In a metaanalysis of 10 trials in 270 patients comparing baclofen and tizanidine, both agents were said to be generally well tolerated; but, no mention was made of ALT abnormalities or hepatotoxicity).
- Afonso Pérez E, Pego Reigosa R, Lancho Seco A, Brañas Fernández F. [Tizanidine-induced toxic hepatitis] Med Clin(Barc) 1999; 112: 478-9. Spanish. [PubMed: 10320966](73 year old man received tizanidine for spasticity after ischemic stroke, developed malaise and jaundice 17 days later [bilirubin 7.2 mg/dL, ALT 616 U/L, Alk P 2810 U/L], resolving within 3 weeks of stopping).
- Saper JR, Winner PK, Lake AE 3rd. An open-label dose-titration study of the efficacy and tolerability of tizanidine hydrochloride tablets in the prophylaxis of chronic daily headache. Headache 2001; 41: 357-68. [PubMed: 11318882](Among 39 patients with frequent headaches treated for 12 weeks with tizanidine, one developed elevated ALT levels [320 U/L] without jaundice or symptoms, which resolved on stopping the drug).
- Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage 2004; 28: 140-75. [PubMed: 15276195](Thorough review of the pharmacology, efficacy and side effects of the muscle relaxants).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to tizanidine or other muscle relaxants).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, one was attributed to chlorzoxazone, but none to tizanidine or other muscle relaxants).
- Henney HR 3rd, Chez M. Pediatric safety of tizanidine: clinical adverse event database and retrospective chart assessment. Paediatr Drugs 2009;11:397-406. [PubMed: 19877725](Summary of tizanidine adverse events in children from off-label use with major focus on somnolence, enuresis and anxiety; ALT elevations mentioned, but they were mild and largely attributed to concurrent anticonvulsant therapy).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. PubMed Citation . [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were due to tizanidine or other muscle relaxants).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver iInjury in the General population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to tizanidine or other muscle relaxants).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases; one case was attributed to verapamil, but none were linked to amlodipine or other calcium channel blockers).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 [0.7%] were attributed to muscle relaxants, including one to trizanidine: 51 year old man developed jaundice 5 weeks after starting tizanidine [bilirubin peak 4.8 mg/dL, ALT 2387 U/L, Alk P 150 U/L, ANA 1:160] and recovered within 2 months of stopping).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- A Case of Tizanidine Withdrawal Syndrome: Features and Management in the Emergency Department.[Cureus. 2023]A Case of Tizanidine Withdrawal Syndrome: Features and Management in the Emergency Department.Morgom M, Sabir DM, Elbashir H, Saeed L, Alamin A, Abuazab Y, Abdelrahman N. Cureus. 2023 Nov; 15(11):e49248. Epub 2023 Nov 22.
- Involvement of imidazoline receptors in the centrally acting muscle-relaxant effects of tizanidine.[Eur J Pharmacol. 2002]Involvement of imidazoline receptors in the centrally acting muscle-relaxant effects of tizanidine.Honda M, Sekiguchi Y, Sato N, Ono H. Eur J Pharmacol. 2002 Jun 12; 445(3):187-93.
- A double-blind comparative trial of new muscle relaxant, tizanidine (DS 103-282), and baclofen in the treatment of chronic spasticity in multiple sclerosis.[Curr Med Res Opin. 1981]A double-blind comparative trial of new muscle relaxant, tizanidine (DS 103-282), and baclofen in the treatment of chronic spasticity in multiple sclerosis.Smolenski C, Muff S, Smolenski-Kautz S. Curr Med Res Opin. 1981; 7(6):374-83.
- Review Chlorzoxazone.[LiverTox: Clinical and Researc...]Review Chlorzoxazone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Tizanidine: neuropharmacology and mechanism of action.[Neurology. 1994]Review Tizanidine: neuropharmacology and mechanism of action.Coward DM. Neurology. 1994 Nov; 44(11 Suppl 9):S6-10; discussion S10-1.
- Tizanidine - LiverToxTizanidine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...