NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tibolone is a synthetic estrogen used for treatment of symptoms of menopause and prevention of osteoporosis. Tibolone has been associated with rare instances of acute, clinically apparent liver injury.
Background
Tibolone (tye' boe lone) is a synthetic estrogen-like steroid hormone which acts as an agonist of multiple type I steroid receptors and thus has weak estrogenic and progestational activities as well as some androgenic properties. The parent compound has no activity, but undergoes tissue-specific metabolism via a sulfatase to an active form which provides estrogenic effects without the need for concurrent progesterone administration. Tibolone also appears to have reduced estrogenic activity in breast tissue. It has been shown to be effective against the symptoms of menopause and in prevention of postmenopausal osteoporosis; it is approved in several European countries, but not the United States. Tibolone is available as 1.25 and 2.5 mg tablets under the brand name Livial. Side effects include vaginal bleeding, abdominal pain, weight gain, bloating, breast tenderness, genital pruritus and vaginitis. Uncommon but potentially severe adverse events include an increased rate of venous thromboses and cerebrovascular ischemic stokes.
Hepatotoxicity
In large scale prospective studies, tibolone has been associated with a low rate of transient serum aminotransferase levels, being greater than 3 times the upper limit of normal in 0.9% of tibolone versus 0.2% of placebo recipients, but instances of clinically apparent acute liver injury were not reported. In Europe, where tibolone has been in clinical use, there have been isolated reports of clinically apparent liver injury arising 6 to 12 months after starting and associated with a hepatocellular pattern of serum enzyme elevations and jaundice. Reported cases were self-limited and resolved within 2 to 6 months of stopping. Immunoallergic features were not present nor were autoantibodies.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of liver injury due to tibolone is not known. The clinically apparent liver injury has not resembled that of the cholestatic jaundice associated with estrogens and has features of idiosyncratic liver injury.
Outcome and Management
The severity of liver injury due to tibolone has varied from mild, transient serum enzyme elevations to moderately severe acute hepatitis. No instances of acute liver failure, death, or chronic hepatitis have been linked to tibolone use. A single instance of vanishing bile duct syndrome was reported after tibolone use in combination with St. John’s wort. Rechallenge studies have not been done.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tibolone – Livial®
DRUG CLASS
Synthetic Estrogen
Product labeling at DailyMed, National Library of Medicine, NIH
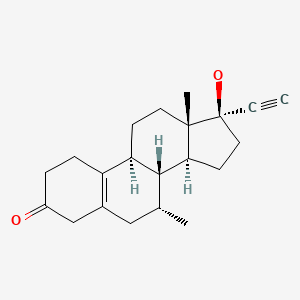
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tibolone | 5630-53-5 | C21-H28-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 02 September 2020
- Zimmerman HJ. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 555-88.(Expert review of hepatotoxicity published in 1999; estrogenic steroids and oral contraceptives are discussed, but not tibolone).
- Chitturi S, Farrell GC. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 605-19.(Review of hepatotoxicity of estrogenic hormones; tibolone is not discussed).
- Isaacs C, Wellstein A, Riegel AT. Hormones and related agents in the therapy of cancer. In, Brunton LL, Hilal-Dandan, R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1237-47.(Textbook of pharmacology and therapeutics).
- Blanco Sampascual S, de Las Heras Niño B, Cabezudo Gil P, Ruiz Eguiluz P, Orive Cura V. Gastroenterol Hepatol. 2002;25:274. [Tibolone-induced hepatotoxicity] Spanish. [PubMed: 11975880](51 year old woman developed jaundice and pruritus 1 year after starting tibolone [bilirubin 43.7 mg/dL, ALT 1800 U/L, Alk P 407 U/L], resolving within 6 months of stopping).
- Swegle JM, Kelly MW. Tibolone: a unique version of hormone replacement therapy. Ann Pharmacother. 2004;38:874–81. [PubMed: 15026563](Review of the structure, pharmacology, mechanism of action and clinical efficacy of tibolone, which has been available in Europe for several years, but not in the U.S.; side effects include vaginal bleeding, abdominal pain, weight gain, bloating, breast tenderness and vaginitis; no mention of hepatotoxicity).
- Rigato I, Cravatari M, Avellini C, Ponte E, Crocè SL, Tiribelli C. Drug-induced acute cholestatic liver damage in a patient with mutation of UGT1A1. Nat Clin Pract Gastroenterol Hepatol. 2007;4:403–8. [PubMed: 17607296](54 year old woman developed jaundice 6 months after starting tibolone and 2 months after starting flavoxate [bilirubin 12.7 mg/dL, ALT 1938 U/L, Alk P 244 U/L], resolving in 2 months of stopping both).
- Bai W, Henneicke-von Zepelin HH, Wang S, Zheng S, Liu J, Zhang Z, Geng L, et al. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: a randomized, double blind, parallel-controlled study versus tibolone. Maturitas. 2007;58:31–41. [PubMed: 17587516](Controlled trial of tibolone vs black cohosh in 244 women with menopausal symptoms; "Adverse events that might have been interpreted as sign of a liver dysfunction did not occur").
- Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, et al. LIFT Trial Investigators. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008;359:697–708. [PMC free article: PMC3684062] [PubMed: 18703472](Controlled trial of tibolone vs placebo in 4538 women with osteoporosis; ALT elevations >3 times ULN occurred in 0.9% on tibolone and 0.2% placebo, no mention of clinically apparent liver injury; the study was stopped because of increased incidence of stroke on tibolone).
- Etogo-Asse F, Boemer F, Sempoux C, Geubel A. Acute hepatitis with prolonged cholestasis and disappearance of interlobular bile ducts following tibolone and Hypericum perforatum (St. John's wort). Case of drug interaction? Acta Gastroenterol Belg. 2008;71:36–8. [PubMed: 18396749](57 year old woman developed jaundice 2 years after starting tibolone and 10 weeks after starting St. John's wort [bilirubin 6.3 rising to 37.0 mg/dL, ALT 424 U/L, Alk P 162 U/L, ANA 1:320], with slow improvement and biopsy indicating bile duct loss; authors suggested an interaction between herbal and tibolone).
- Chen J, Gao H, Li Q, Cong J, Wu J, Pu D, Jiang G. Efficacy and safety of remifemin on peri-menopausal symptoms induced by post-operative GnRH-a therapy for endometriosis: a randomized study versus tibolone. Med Sci Monit. 2014;20:1950–7. [PMC free article: PMC4211309] [PubMed: 25321621](Among 116 Chinese women with endometriosis undergoing gonadotrophin releasing hormone therapy and treated with either remifemin [black cohosh] or tibolone, menopausal symptoms and hot flashes/sweating scores increased in both groups to a similar degree and there were no significant changes in ALT, AST or creatinine levels).
- Chen JM, Gao HY, Ding Y, Yuan X, Wang Q, Li Q, Jiang GH. Efficacy and safety investigation of Kuntai capsule for the add-back therapy of gonadotropin releasing hormone agonist administration to endometriosis patients: a randomized, double-blind, blank- and tibolone-controlled study. Chin Med J (Engl). 2015;128:427–32. [PMC free article: PMC4836241] [PubMed: 25673440](Among 30 Chinese women with endometriosis undergoing gonadotrophic releasing hormone therapy, menopausal symptoms and hot flashes were less in those receiving tibolone and a Traditional Chinese medication [Kuntai] than those on no therapy and there were no significant rises in ALT or AST levels in any group).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to tibolone which was not approved for use in the United States).
- Macedo G, Silva M, Vilas-Boas F, Lopes S, Peixoto A, Carneiro F. Tibolone-induced acute hepatitis: Well-known drug, little-known complication. Gastroenterol Hepatol. 2017;40:298–300. [PubMed: 27093896](45 year old woman was found to have asymptomatic elevation of liver tests 6 months after starting tibolone for menopausal symptoms [bilirubin normal, ALT 600 U/L, Alk P normal], which fell into the normal range within 3 weeks of stopping).
- Alshehre SM, Duffy S, Jones G, Ledger WL, Metwally M. A prospective, single-centre, single-arm, open label study of the long term use of a gonadotropin releasing hormone agonist (Tin SR, 11.25 mg) in combination with Tibolone add-back therapy in the management of chronic cyclical pelvic pain. Reprod Biol Endocrinol. 2020;18:28. [PMC free article: PMC7155249] [PubMed: 32290838](Among 27 women receiving gonadotrophin releasing hormone agonists as therapy for cyclic pelvic pain treated with tibolone, there were no serious adverse events and although ALT levels rose slightly, they remained in the normal range).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Tibolone: a unique version of hormone replacement therapy.[Ann Pharmacother. 2004]Review Tibolone: a unique version of hormone replacement therapy.Swegle JM, Kelly MW. Ann Pharmacother. 2004 May; 38(5):874-81. Epub 2004 Mar 16.
- Tibolone: clinical recommendations and practical guidelines. A report of the International Tibolone Consensus Group.[Maturitas. 2005]Tibolone: clinical recommendations and practical guidelines. A report of the International Tibolone Consensus Group.Kenemans P, Speroff L, International Tibolone Consensus Group. Maturitas. 2005 May 16; 51(1):21-8.
- Molecular analysis of human endometrium: short-term tibolone signaling differs significantly from estrogen and estrogen + progestagen signaling.[J Mol Med (Berl). 2007]Molecular analysis of human endometrium: short-term tibolone signaling differs significantly from estrogen and estrogen + progestagen signaling.Hanifi-Moghaddam P, Boers-Sijmons B, Klaassens AH, van Wijk FH, den Bakker MA, Ott MC, Shipley GL, Verheul HA, Kloosterboer HJ, Burger CW, et al. J Mol Med (Berl). 2007 May; 85(5):471-80. Epub 2007 Jan 17.
- Tibolone improves depression in women through the menopause transition: A double-blind randomized controlled trial of adjunctive tibolone.[J Affect Disord. 2018]Tibolone improves depression in women through the menopause transition: A double-blind randomized controlled trial of adjunctive tibolone.Kulkarni J, Gavrilidis E, Thomas N, Hudaib AR, Worsley R, Thew C, Bleeker C, Gurvich C. J Affect Disord. 2018 Aug 15; 236:88-92. Epub 2018 Apr 24.
- Review Effects of tibolone on the breast of postmenopausal women.[Taiwan J Obstet Gynecol. 2007]Review Effects of tibolone on the breast of postmenopausal women.Wang PH, Cheng MH, Chao HT, Chao KC. Taiwan J Obstet Gynecol. 2007 Jun; 46(2):121-6.
- Tibolone - LiverToxTibolone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...