NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tetracycline is an oral, broad-spectrum antibiotic used to treat mild-to-moderate infections due to susceptible microbial organisms. High doses of several forms of tetracycline given intravenously have been associated with acute fatty liver that can be severe and result in liver failure and death. Oral tetracycline use has been rarely and not very convincingly linked to acute hepatic injury.
Background
Tetracycline is an oral, broad-spectrum antibiotic and semisynthetic derivative of Streptomyces actinobacteria. Tetracycline acts by inhibition of protein synthesis by binding to the 30S subunit of microbial ribosomes. Human cells are less susceptible to this inhibition. Tetracycline was first approved for use in the United States in 1957 and was one of several oral tetracyclines used at that time (oxytetracycline, chlortetracycline), many of which are no longer available or are used in veterinary medicine only. More modern forms of tetracycline include doxycycline and minocycline which are much more commonly used and have similar indications. Currently, tetracycline is most frequently used for upper respiratory and skin and soft tissue infection and more than 2 million prescriptions are filled yearly. Chronic therapy with tetracycline is effective in ameliorating acne, but because of their better absorption and tissue penetration, minocycline and doxycycline have largely replaced tetracycline for this indication. Tetracycline is also active against infections with several rickettsial, spirochetal, chlamydial and mycoplasmas infections and are often used for therapy of nonspecific urethritis and several Rickettsia diseases, such as Rocky Mountain spotted fever and Lyme disease. Tetracycline is available in multiple generic forms as capsules or tablets of 250 and 500 mg and generally recommended in doses of 250 to 500 mg three to four times daily for 7 to 30 days. Chronic therapy is typical for therapy of acne. Pediatric formulations as oral suspension are also available. Parenteral tetracycline is no longer used. Common side effects include gastrointestinal upset, nausea, poor appetitle, diarrhea, glossitis, rash and hypersensitivity reactions. Tetracycline can cause staining of developing teeth (in children or when taken by a pregnant mother).
Hepatotoxicity
High doses of intravenous tetracycline can induce fatty liver disease and may result in severe hepatic dysfunction, acute liver failure and death. This syndrome is more common among pregnant women, largely during the last trimester or early postpartum period. However, instances of acute fatty liver attributed to intravenous tetracycline have been reported in nonpregnant women and in men and even in children. The injury is characterized by onset of weakness, fever, fatigue, nausea and abdominal pain after 3 to 10 days of therapy. Laboratory tests show minimal-to-moderate elevations in serum aminotransferase and alkaline phosphatase levels with mild jaundice, but presence of hyperammonemia and coagulopathy. Pancreatitis, renal dysfunction and lactic acidosis are also common, although not always specifically sought. The syndrome may be reversible if tetracycline is stopped promptly, but it is usually recognized late and can progress to multiorgan failure and death despite stopping the agent. This syndrome also occurs with high doses of intravenous doxycycline and minocycline, but quite rarely. This syndrome is rarely seen currently as tetracycline is no longer available in parenteral form and the use of intravenous tetracyclines has been superseded by availability of safer, better tolerated and more effective broad spectrum antibiotics.
Oral tetracycline has been associated with rare instances of acute liver injury, but the association with tetracycline use as opposed to other agents being taken has not always been very well shown. Despite frequency of its use, oral tetracycline remains a very rare cause of liver injury. In contrast, cases of doxycycline and minocycline induced liver disease are well described. Rare instances of acute fatty liver have been attributed to oral tetracycline, particularly when given to pregnant women in high doses. Currently, tetracycline is considered contraindicated in pregnant women, particularly during the last trimester.
Likelihood score: A[HD] (well known cause of clinically apparent liver injury but usually when given in high doses intravenously).
Mechanism of Injury
The hepatic injury from intravenous forms of tetracycline was likely due to mitochondrial injury due to inhibition of mitochondrial protein synthesis, although another possibility is that tetracycline interferes with fat metabolism in hepatocytes. The injury is less idiosyncratic than direct, varying in frequency and severity based upon pharmacokinetics and host metabolic factors (age, pregnancy, drug accumulation, and other factors affecting mitochondrial function). The rare idiosyncratic forms of liver injury from tetracycline are of unknown cause, but are likely to be immunoallergic.
Outcome and Management
Acute fatty liver due to tetracycline has a high fatality rate and is best managed with intensive care and attention to multiorgan support. Lactic acidosis should be treated with intravenous glucose (20% infusions) and bicarbonate. Idiosyncratic acute liver injury from tetracycline is rare and usually resolves rapidly once the agent is withdrawn. An instance of chronic cholestasis with vanishing bile duct syndrome has been reported, although the patient had received several medications that might have accounted for the hepatic injury.
Drug Class: Antiinfective Agents, Tetracyclines
CASE REPORT
Case 1. Liver failure from intravenous tetracycline.
[Modified from: Robinson MJ, Rywlin AM. Tetracycline-associated fatty liver in the male. Report of an autopsied case. Am J Dig Dis 1970; 15: 857-62. PubMed Citation]
A 72 year old man treated for cystitis and diverticulitis with 10 days of iv tetracycline developed lactic acidosis and multiorgan failure. The patient had no previous history of liver disease or risk factors for hepatitis. When initially admitted to the hospital, he had fever, abdominal pain, leukocytosis and an abnormal urinalysis, but liver tests were normal (Table). He was treated with hydration and iv tetracycline in doses of 3 grams daily. His fever improved slowly, but by day 4, liver tests were mildly abnormal and they had worsened by day 8. Colistin was added on day 6, and chloramphenicol was substituted for tetracycline on day 10. The following day, he developed progressive restlessness, stupor and respiratory failure. He had renal failure and severe acidosis and he died within hours of attempting resuscitation and dialysis. Autopsy showed an enlarged fatty liver with minimal inflammation and no obvious hepatocellular necrosis. Fat was also identified in renal tubular cells. There was marked ascites and hemorrhagic pancreatitis.
Key Points
| Medication: | Tetracycline (3 g iv daily for 10 days) |
| Pattern: | Hepatocellular |
| Severity: | 5+ (death from hepatic failure and lactic acidosis) |
| Latency: | 4 days |
| Recovery: | Fatal |
| Other medications: | Colistin and chloramphenicol (after onset of liver injury) |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P* (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Pre | 32 | 8 | 0.3 | Started iv tetracycline |
| 4 days | 190 | 10 | 2.4 | BUN 39 mg/dL | |
| 8 days | 530 | 11.5 | 2.2 | Colistin added im q 12 hr | |
| 10 days | 0 | Tetracycline stopped; chloramphenicol started. | |||
| 11 days | 1 day | 1550 | 15 | 6.8 | BUN 105; CO2 11 mEq/L |
| Increasing stupor and respiratory failure, hemodialysis and resuscitative measures failed. | |||||
| Normal Values | <40 | <13 | <1.2 | ||
* King Armstrong units
Comment
Acute fatty liver caused by tetracycline was initially thought to occur only in pregnant women during the last trimester, but was subsequently reported to occur at other times during pregnancy, after delivery, in nonpregnant women, in men and even in children. The syndrome typically occurs after 3 to 10 days of relatively high doses of im or iv tetracycline. The case above is typical in demonstrating that the precipitous onset of symptoms is a late phenomenon and has a grim prognosis; symptoms are preceded by several days of worsening liver injury, although laboratory tests may be only mildly or moderately abnormal. Acute fatty liver is accompanied by minimal hepatic inflammation and liver cell necrosis and is a syndrome of mark mitochondrial failure, early appearance of hepatic synthetic dysfunction and lack of ATP to drive normal metabolism. The injury is not confined to the liver, and renal failure and pancreatitis are common. As in this case, the immediate cause of death is typically multiorgan failure due to lactic acidosis or pancreatitis.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tetracycline – Generic, Sumycin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
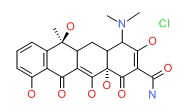
| Tetracycline Hydrochloride | 64-75-5 | C22-H24-N2-O8.Cl-H |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 10 June 2017
- Zimmerman HJ. Tetracyclines. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p. 599-602.(Expert review of tetracycline and liver injury published in 1999; the tetracyclines cause two forms of drug induced liver injury, microvesicular fat and liver failure occurring after 4-10 days with high does of parenteral tetracyclines and an idiosyncratic liver injury that occurs with the oral agents, doxycycline causing a cholestatic and minocycline a hepatocellular injury which may be associated with autoimmune features).
- Moseley RH. Tetracyclines. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 468.(Expert review of tetracycline induced liver injury mentions that the hepatotoxicity of intravenous tetracycline is of historic interest only as it is no longer given parenterally; both doxycycline and minocycline have been associated with idiosyncratic liver injury).
- MacDougall C, Chambers HF. Tetracyclines and glycylcyclines. Protein synthesis inhibitors and miscellaneous antibacterial agents. In, Brunton LL, Chabner KA, Knollman KC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1521-6.(Textbook of pharmacology and therapeutics).
- Lepper MH, Wolfe CK, Zimmerman HJ, Cladwell ER Jr, Spies HW, Dowling HF. Effect of large doses of aureomycin on human liver. Arch Intern Med 1951; 88: 271-83. [PubMed: 14856456](Description of hepatotoxicity among 101 patients treated with aureomycin over a 30 month period; 7 had clinically apparent liver injury, all received high doses [>3 g] intravenously with onset of vomiting and mental changes followed by jaundice after 3-12 days; autopsy showed fatty liver).
- Rutenburg AM, Pinkes S. The hepatotoxicity of intravenous aureomycin. N Engl J Med 1952; 247: 797-800. [PubMed: 13002619](Serum bilirubin rose in some patients given iv aureomycin and some became “jaundiced”, but may have been partially an artifact of colorimetric method of bilirubin detection).
- Domz CA, McNamara DH, Holzapfel HF. Tetracycline provocation in lupus erythematosus. Ann Intern Med 1959; 50: 1217-26. [PubMed: 13650436](3 cases of apparent worsening of systemic lupus erythematosus after course of oxytetracycline for incurrent infection; no hepatic component mentioned).
- Ticktin HE, Zimmerman HJ. Hepatic dysfunction and jaundice in patients receiving triacetyloleandomycin. N Engl J Med 1962; 267: 964-8. Not in PubMed.(50 “mental defectives or juvenile delinquents” were given triacetyloleandomycin [1 g orally per day] for 3-4 weeks with close monitoring, BSP retention increased in 54% by week 2, AST in 34%, ALT in 8%, 12% had symptoms, 4% jaundice; liver biopsy showed mixed cholestatic-hepatic picture in 2; rechallenge led to increases in AST and bilirubin in 2 within 1-2 days; all ultimately resolved).
- Schultz JC, Adamson JS Jr, Workman WW, Norman TD. Fatal liver disease after intravenous administration of tetracycline in high dosage. N Engl J Med 1963; 269: 999-1004. [PubMed: 14059734](Initial dramatic report of acute fatty liver due to iv tetracycline; a rapidly fatal multisystem disease appeared in 6 pregnant women in their last trimester or early postpartum period after 3 to 5 days of relatively high doses of iv tetracycline; all developed jaundice [bilirubin 4.9-12.5 mg/dL] with mild AST [72-170 U/L] and Alk P [9-17 King Armstrong U/L] elevations, prolongation of protime, acidosis and renal dysfunction. Autopsy showed foamy hepatocytes, fat by oil red O stain and minimal hepatocyte necrosis).
- Tetracycline hepatotoxicity. Br Med J 1964; 2: 1545-6. [PMC free article: PMC1817573] [PubMed: 14211749](Editorial on the recently described syndrome of acute fatty liver in pregnant women receiving iv tetracycline).
- Dowling HF, Lepper MH. Hepatic reactions to tetracycline. JAMA 1964; 188: 307-9. [PubMed: 14114004](Review of literature on acute fatty liver due to tetracycline and recommendations on its use: avoiding doses >2 g/day and cautious use in women in last trimester of pregnancy).
- Horwitz ST, Marymont JH Jr. Fatal liver disease during pregnancy associated with tetracycline therapy. Report of a case. Obstet Gynecol 1964; 23: 826-9. [PubMed: 14168241](After complicated Caesarian and emergency hysterectomy with shock and bleeding, 41 year old woman received tetracycline given iv for 4 days, then orally to day 8; on day 10 she developed fever, acidosis and ileus, dying on day 16 with multiorgan failure and jaundice; autopsy showed fatty liver).
- Norman TD, Schultz JC, Hoke RD. Fatal liver disease following the administration of tetracycline. South Med J 1964; 57: 1038-42. [PubMed: 14179316](Further analysis of 6 cases of acute fatty liver after tetracycline in pregnant women with histological changes).
- Whalley PJ, Adams RH, Combes B. Tetracycline toxicity in pregnancy. Liver and pancreatic dysfunction. JAMA 1964; 189: 357-62. [PubMed: 14160508](Five cases of acute fatty liver with azotemia and pancreatitis after 4-17 days of iv or oral therapy 1-2 g/day: bilirubin 1.2-18.8 mg/dL; AST 34-780 U/L; Alk P 8.6-22 BU/L; 1 case fatal. The anicteric case had pancreatitis and was attributed to oral drug, but had fat on liver biopsy).
- Brewer T. Tetracycline hepatotoxicity. Br Med J 1965; 1: 995. [PMC free article: PMC2165688] [PubMed: 14260641](Letter questioning role of tetracycline in acute fatty liver).
- Finn WF, Horwitz ST. Maternal death due to fatty metamorphosis of liver following tetracycline therapy. N Y State J Med 1965; 65: 662-7. [PubMed: 14251580](After Caesarian section, followed by hysterectomy and pneumonia, 41 year old woman received iv tetracycline for 4 days, followed by oral and then iv drug again, developing shock and jaundice; autopsy showed fatty liver without necrosis).
- Ichida F, Watanabe K, Ishiwara S, Inoue K, Tanabe Y. [A case of cholestatic jaundice due to tetracycline-triacetyloleandomycin] Nippon Naika Gakkai Zasshi 1965; 54:142-7. Japanese. [PubMed: 5294320]
- Kunelis CT, Peters JL, Edmondson HA. Fatty liver of pregnancy and its relationship to tetracycline therapy. Am J Med 1965; 38: 359-77. [PubMed: 14266828](Classical clinical and histologic description of syndrome in 12 pregnant women, ages 19-31, given tetracycline for 3 to 12 days [total dose 1.5 to 14 g] after 16-39 weeks of pregnancy [bilirubin 12-19.8 mg/dL, ALT 24-210 U/L, AST 36-760 U/L, lactic acid high in 3/3 patients], 67% fatal, often accompanied by multiorgan failure, pancreatitis and shock).
- Tapp E, Carroll R. Tetracycline accumulation in toxic liver damage. J Pathol Bacteriol 1965; 89: 715-21. [PubMed: 14320315](Animal studies showing accumulation of tetracycline in damaged hepatocytes, particularly with high levels of calcium).
- Winterling AN, Goldman RL. Hepatic and renal lesions in a case of tetracycline toxicity during long-term estrogen therapy after orchiectomy. Calif Med 1965; 102: 314-6. [PMC free article: PMC1515729] [PubMed: 14291456](66 year old man post-orchiectomy for prostate cancer developed sudden onset of fever, lactic acidosis and mild jaundice [bilirubin 2.9 mg/dL] after 8 days of iv tetracycline [17 g], subsequently died after emergency laparotomy; autopsy showed fatty liver).
- Wruble LD, Ladman AJ, Britt LG, Cummins AJ. Hepatotoxicity produced by tetracycline overdosage. JAMA 1965; 192: 6-8. [PubMed: 14262279](56 year old woman with cecal volulus developed jaundice [bilirubin 7.8 mg/dL, AST 98 U/L, Alk P normal] 2 days after receiving 8 g of tetracycline, subsequently progressing to liver failure and death; autopsy showed fatty liver).
- Wruble LD, Cummins AJ. Tetracycline and fatty liver. Am J Dig Dis 1965; 10: 742-4. [PubMed: 14316764](Editorial on tetracycline induced acute fatty liver with discussion of mechanisms focusing on mitochondrial injury).
- Tetracyclines and the liver in pregnancy. Lancet 1966; 1: 357-8. [PubMed: 4159865](Editorial on the history of acute fatty liver of pregnancy, first described by Sheehan in 1940 and later linked to high dose iv tetracycline in pregnancy, but also in nonpregnant women and in men).
- Allen ES, Brown WE. Hepatic toxicity of tetracycline in pregnancy. Am J Obstet Gynecol 1966; 95: 12-8. [PubMed: 5934994](Prospective study done in 18 pregnant women who underwent liver biopsy and then were given 1-6 g of tetracycline [n=9] or oxytetracycline [n=9] intravenously for 3 days, at which time liver biopsy was repeated; increased AST [16 to 134 U/L] in 1, increase in fat in liver in most, but only detectable by oil red O stain, not by usual stains).
- Davis JS, Kaufman RH. Tetracycline toxicity. A clinicopathologic study with special reference to liver damage and its relationship to pregnancy. Am J Obstet Gynecol 1966; 95: 523-9. [PubMed: 5939019](6 cases of acute fatty liver, 1 man and 5 women [4 pregnant] given intravenous tetracycline for 9-10 days [3-6 g] [bilirubin 3-12.5 mg/dL, ALT 34-310 U/L], all acidotic and all died, autopsy showing fatty liver).
- Schiffer MA. Fatty liver associated with administration of tetracycline in pregnant and nonpregnant women. Am J Obstet Gynecol 1966; 96: 326-32. [PubMed: 5919490](Six cases of acute fatty liver from tetracycline, 2 in pregnancy at 34- and 40 weeks; 4 in nonpregnant women, but clinical course not described).
- Aach R, Kissane J eds. Clinicopathologic conference. A seventeen-year-old girl with fatty liver of pregnancy following tetracycline therapy. Am J Med 1967; 43: 274-83. [PubMed: 6034958](Clinicopathologic conference describing 17 year old pregnant woman who developed suspected pyelonephritis, was treated with im and oral tetracycline and 8 days later developed multiorgan failure, lactic acidosis and died despite induced delivery of a stillborn child).
- Lewis M, Schenker S, Combes B. Studies on the pathogenesis of tetracycline-induced fatty liver. Am J Dig Dis 1967; 12: 429-38. [PubMed: 6026422](Rats given high doses of iv tetracycline develop fat in liver with higher concentrations in pregnant rats, despite similar serum and hepatic concentrations of drug).
- Peters RL, Edmondson HA, Mikkelsen WP, Tatter D. Tetracycline-induced fatty liver in nonpregnant patients. A report of six cases. Am J Surg 1967; 113: 622-32. [PubMed: 6021433](Classical description of clinical and histologic features of tetracycline induced acute fatty liver in nonpregnant patients; 6 nonpregnant women, ages 18-62, given iv tetracycline for 3-13 days [9-26 g] presenting with jaundice and lethargy; peak AST 52-960 U/L; bilirubin 2.7-7.4 mg/dL, often with complex and critical course, pancreatitis and renal failure, acidosis; fatty liver on autopsy).
- Damjanov I, Arnold R, Faour M. Tetracycline toxicity in a nonpregnant woman. JAMA 1968; 204: 934. [PubMed: 5694615](20 year old woman given iv tetracycline for 8 days after second of two gall bladder operations developed sudden onset of renal and hepatic failure [bilirubin 4.2 mg/dL, ALT 620 U/L], autopsy showed fatty liver and acute tubular necrosis).
- Hansen CH, Pearson LH, Schenker S, Combes B. Impaired secretion of triglycerides by the liver; a cause of tetracycline-induced fatty liver. Proc Soc Exp Biol Med 1968; 128: 143-6. [PubMed: 5656682](Tetracycline induces fatty liver in rats at least in part due to inhibition of hepatic secretion of triglycerides).
- Breitenbucher RB, Crowley LV. Hepatorenal toxicity of tetracycline. Minn Med 1970; 53: 949-55. [PubMed: 5470245](Seven patients with acute fatty liver from tetracycline, arising 5-11 days after starting iv tetracycline [6-19 g] with complex courses, but fatty liver on autopsy in all).
- Kunin CM. Hepatorenal toxicity of tetracycline. Minn Med 1971; 5: 532-3. [PubMed: 5564809](Editorial in response to Breitenbucher questioning whether tetracycline was responsible for the clinical syndrome and attributing any injury to overdosage with tetracycline).
- Robinson MJ, Rywlin AM. Tetracycline-associated fatty liver in the male. Report of an autopsied case. Am J Dig Dis 1970; 15: 857-62. [PubMed: 5459744](72 year old man given 10 days of iv tetracycline [30 g total] developed worsening liver injury followed by hepatic and renal failure and severe acidosis [bilirubin 6.8 mg/dL, AST 1550 KU/L, Alk P 15 KA U/L]; autopsy showed fatty liver, fatty vacuolization of renal tubular cells and pancreatitis).
- Breen KJ, Perkins KW, Mistilis SP, Shearman R. Idiopathic acute fatty liver of pregnancy. Gut 1970; 11: 822-5. [PMC free article: PMC1553146] [PubMed: 5485832](23 year old woman who survived acute fatty liver of pregnancy; onset day after spontaneous labor and stillborn delivery [bilirubin 7.9 mg/dL, Alk P 70 King Armstrong U/L, AST 67 U/L, prothrombin index 50%], liver biopsy showed fatty liver, improved with dextrose administration).
- Combes B, Whalley PJ, Adams RH. Tetracycline and the liver. Prog Liver Dis 1972; 4: 589-96. [PubMed: 4569011](Review of hepatotoxicity of tetracycline, including studies of pathogenesis).
- Pride GL, Cleary RE, Hamburger RJ. Disseminated intravascular coagulation associated with tetracycline-induced hepatorenal failure during pregnancy. Am J Obstet Gynecol 1973; 115: 585-6. [PubMed: 4685515](23 year old pregnant woman developed jaundice [bilirubin 7.8 mg/dL, AST 346 U/L, Alk P 38 U/L] and oliguria 2 weeks after 2 im injections of tetracycline who delivered a normal child, but then had self-limiting disseminated intravascular coagulation and hemolytic anemia after delivery).
- Whalley PJ, Combes B. Letter: Tetracycline toxicity. Am J Obstet Gynecol 1974; 118: 1149-51. [PubMed: 4817657](Letter in response to article by Pride et al. questioning the role of tetracycline because of dose and timing).
- Lloyd-Still JD, Grand RJ, Vawter GF. Tetracycline hepatotoxicity in the differential diagnosis of postoperative jaundice. J Pediatr 1974; 84: 366-70. [PubMed: 4590715](3 children [ages 2, 3 and 10 years; all girls] with chronic urinary tract disease developed jaundice and liver failure after surgery and several days of iv tetracycline; 2 autopsies and one liver biopsy showed fatty liver).
- Taneja OP, Grover NK, Thakur LC, Bhatia VN. Effects of blood levels of tetracycline and oxytetracycline on hepatic and renal functions in normal subjects. Chemotherapy 1974; 20: 201-11. [PubMed: 4413117](Mild increases in ALT and AST found in 20 healthy volunteers given oxytetracycline or tetracycline [1 g/day orally] for 5 days; no clinical symptoms or jaundice).
- Schenker S. Tetracycline hepatotoxicity. A review. Mater Med Pol 1976; 8: 173-6. [PubMed: 790035]
- Wenk RE, Gebhardt FC, Bhagavan BS, Lustgarten JA, McCarthy EF. Tetracycline-associated fatty liver of pregnancy, including possible pregnancy risk after chronic dermatologic use of tetracycline. J Reprod Med 1981; 26: 135-41. [PubMed: 7230149](Two cases of acute fatty liver over a 15 year period and 60,000 deliveries; one woman received tetracycline for 3 days, the second had no history of its use, but had tetracycline detected in bony tissue suggestive of chronic use).
- Böcker R, Estler CJ, Müller S, Pfandzelter C, Spachmüller B. Comparative evaluation of the effects of tetracycline, rolitetracycline and doxycycline on some blood parameters related to liver function. Arzneimittelforschung 1982; 32: 237-41. [PubMed: 7200783](Minocycline caused a dose dependent rise in AST and increase in triglycerides, but no hepatic histological changes in mice; similar findings were previously reported with tetracycline).
- Carson JL, Strom BL, Duff A, et al. Acute liver disease associated with erythromycins, sulfonamides, and tetracyclines. Ann Intern Med 1993; 119 (7 Pt 1): 576-83. [PubMed: 8363168](Case control study using Medicaid billing results between 1980-87 found 107 cases of hospitalization for unexplained hepatitis, odds ratios for erythromycin 5.2; sulfonamides 11.4; tetracyclines 5.2; total of 5 cases exposed to tetracycline, doxycycline or minocycline).
- Hunt CM, Washington K. Tetracycline-induced bile duct paucity and prolonged cholestasis. Gastroenterology 1994; 107: 1844-7. [PubMed: 7958700](Two cases of severe and prolonged cholestatic hepatitis and bile duct paucity after oral tetracyclines; 37 year old woman developed jaundice 2 days after 3 day course of doxycycline with prolonged cholestasis [peak bilirubin 30 mg/dL], but ultimate recovery; 63 year old woman developed jaundice 6 weeks after a 2 week course of tetracycline [bilirubin 11.8 mg/dL, ALT 245 U/L], with prolonged jaundice [peak bilirubin 29.5 mg/dL) and persistence of enzyme elevations for >3 years [Alk P 631 U/L, ALT 97 U/L]).
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med 1992; 232: 133-8. [PubMed: 1506809](Adverse drug reaction reports between 1978 and 1987 in Denmark; no tetracycline is mentioned as a cause).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years’ experience. N Z Med J 1996; 109: 315-9. [PubMed: 8816722](Adverse drug reaction reports identified 943 liver injuries over 21 years in New Zealand; triacetyloleandomycin accounted for 21 cases [2.1%] and minocycline for at least 4).
- Tham SN, Kwok YK, Chan HL. Cross-reactivity in fixed drug eruptions to tetracyclines. Arch Dermatol 1996; 132: 1134-5. [PubMed: 8795565](Among 9 Chinese patients with a fixed drug eruption due to tetracycline, all had recurrence on doxycycline and 3 on minocycline; no mention of liver involvement).
- Shapiro LE, Knowles SR, Shear NH. Comparative safety of tetracycline, minocycline, and doxycycline. Arch Dermatol 1997; 133: 1224-30. [PubMed: 9382560](Review of toxicity of tetracyclines from literature and a Canadian database; minocycline had the highest rates of adverse events, but all were relatively safe: no lupus-like syndrome associated with doxycycline or tetracycline).
- Westermann GW, Böhm M, Bonsmann G, Rahn KH, Kisters K. Chronic intoxication by doxycycline use for more than 12 years. J Intern Med 1999; 246: 591-2. [PubMed: 10620103](38 year old man took large doses of doxycycline chronically and developed repeated bouts of mild jaundice [bilirubin 3.4-5.0 mg/dL, ALT 44-118 U/L, Alk P 466 U/L] and weakness, skin discoloration and heart block, resolving upon stopping).
- Sturkenboom MC, Meier CR, Jick H, Stricker BH. Minocycline and lupuslike syndrome in acne patients. Arch Intern Med 1999; 159: 493-7. [PubMed: 10074958](Case control study in 27,688 acne patients in UK database, risk of lupus increased 8.5 fold in minocycline treated patients, not with other tetracyclines, typically long term therapy; liver involvement not mentioned; more common in women, absolute risk low).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, none of which were attributed to minocycline, doxycycline or tetracycline).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-1101. [PubMed: 16165719](Among 103 cases of fulminant drug induced liver injury reported to a Swedish registry between 1966 and 2002, one case was attributed to doxycycline, but no other tetracycline mentioned).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology 2005; 129: 512-21. [PubMed: 16083708](Reports to a Spanish network found 461 cases of drug induced liver disease; no tetracycline was listed among the top 20 agents implicated [at least 5 cases]).
- Galan MV, Potts JA, Silverman AL, Gordon SC. The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center. J Clin Gastroenterol 2005; 39: 64-7. [PubMed: 15599214](Ten year experience of 96 patients with acute liver injury, 64 were due to viral hepatitis and 32 to drugs; minocycline accounted for 4 cases and ranked second in frequency [7 from amiodarone and 4 from amoxicillin/clavulanate]).
- Chamberlain MC, Schwarzenberg SJ, Akin EU, Kurth MH. Minocycline-induced autoimmune hepatitis with subsequent cirrhosis. J Pediatr Gastroenterol Nutr 2006; 42: 232-5. [PubMed: 16456421](15 year old boy developed signs of cirrhosis after 1 year of minocycline therapy [bilirubin 2.5 mg/dL, ALT 67-83 U/L, Alk P normal, ANA 1:40, anti-DNA positive], treated with 6-mercaptopurine and prednisone with only partial response and found to have cirrhosis on follow up liver biopsy).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis 2006; 38: 33-8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports – 89% from the US; the list of most 21 most commonly implicated drugs did not include a tetracycline).
- Margolis DJ, Hoffstad O, Bilker W. Association or lack of association between tetracycline class antibiotics used for acne vulgaris and lupus erythematosus. Br J Dermatol 2007; 157: 540-6. [PubMed: 17596147](Analysis of UK database on 97,694 subjects with acne, 25% received minocycline, 16% doxycycline and 45% other tetracyclines. Hazard ratio for lupus-like syndrome was 3.11 for minocycline; no increased risk or association with doxycycline or other tetracyclines).
- Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case-control study. J Clin Pharm Ther 2007; 32: 483-7. [PubMed: 17875115](Analysis of 2 years of Medicaid claims in California found 3377 cases of “hepatotoxicity”; 20 had received tetracycline <45 days before onset; only 4 controls had: adjusted odds ratio 3.7; not elevated for doxycycline; this despite safety record of oral tetracyclines and known hepatotoxicity of doxycycline).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, minocycline accounted for 3 cases, doxycycline for 3 cases and tetracycline was listed as a secondary possible cause for one).
- Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol 2009; 43: 787-90. [PubMed: 19262406](3 cases of minocycline hepatotoxicity; 16 year olds with onset of jaundice 2, 13 and 24 months after starting minocycline for acne [bilirubin 19.6, 9.1 and 9.5 mg/dL, ALT 1282, 2306 and 2056 U/L, ANA 1:160-1:640], liver biopsies showing hepatitis and fibrosis; all treated with corticosteroids, 2 requiring prolonged therapy).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database containing 9036 hepatic adverse drug reactions in children includes 117 cases attributed to minocycline, but no other tetracycline listed in the top 40 causes).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 2010; 105: 2396-404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were due to tetracyclines).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 25 to antituberculosis agents, including 15 to isoniazid alone [ranking first], 6 to isoniazid combined with other agents, 3 to rifampin and pyrazinamide, and 1 to dapsone).
- Leitner JM, Graninger W, Thalhammer F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical. Infection 2010; 38: 3-11. [PubMed: 20107858](Review of hepatotoxicity of antibiotics; mentions that hepatotoxicity from oral tetracycline is rare ~1.5 cases per million prescriptions, whereas minocycline has been associated with either an immediate reaction with eosinophilia, dermatitis and enzyme elevations or a delayed autoimmune hepatitis-like syndrome).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to a tetracyclinel).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 37 of which were attributed to an antibiotic, but none to a tetracycline).
- Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Sarganas G, Kreutz R, et al. Drug-induced liver injury: results from the hospital-based Berlin Case-Control Surveillance Study. Br J Clin Pharmacol 2015; 79: 988-99. [PMC free article: PMC4456131] [PubMed: 25444550](Among 76 inpatients with hepatitis of uniknown cause enrolled in a prospective case-cohort surveillance study between 2002 and 2011, one was attributed to doxycycline, but no other tetracycline was implicated).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics including 28 [3%] to minocycline and 4 [0.4%] to doxycycline).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Eravacycline.[LiverTox: Clinical and Researc...]Review Eravacycline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline.[Gastroenterology. 1991]Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline.Lichtman SN, Keku J, Schwab JH, Sartor RB. Gastroenterology. 1991 Feb; 100(2):513-9.
- Review Omadacycline.[LiverTox: Clinical and Researc...]Review Omadacycline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Sarecycline.[LiverTox: Clinical and Researc...]Review Sarecycline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Bicyclol attenuates tetracycline-induced fatty liver associated with inhibition of hepatic ER stress and apoptosis in mice.[Can J Physiol Pharmacol. 2016]Bicyclol attenuates tetracycline-induced fatty liver associated with inhibition of hepatic ER stress and apoptosis in mice.Yao XM, Li Y, Li HW, Cheng XY, Lin AB, Qu JG. Can J Physiol Pharmacol. 2016 Jan; 94(1):1-8. Epub 2015 Sep 22.
- Tetracycline - LiverToxTetracycline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...