NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The vesicular monoamine transporter type 2 (VMAT2) inhibitors are agents that cause a depletion of neuroactive peptides such as dopamine in nerve terminals and are used to treat chorea due to neurodegenerative diseases (such as Huntington chorea) or dyskinesias due to neuroleptic medications (tardive dyskinesia). As of 2019, three VMAT2 inhibitors have become available in the United States for management of dyskinesia syndromes, each with a somewhat different spectrum of approved indications: tetrabenazine (Xenazine and generics: 2008), deutetrabenazine (Austedo: 2017) and valbenazine (Ingressa: 2017). The VMAT2 inihibitors have not been associated with serum enzyme elevations during therapy or linked to instances of clinically apparent liver injury, but they have had limited general clinical use.
Tetrabenazine
Background
Tetrabenazine (tet" ra ben' a zeen) is an inhibitor of synaptic vesicular monoamine transporter 2 (VMAT2), the inhibition of which causes a depletion of neuroactive monoamines (serotonin, norepinephrine and particularly dopamine) in nerve terminals. The reduction in these active neurotransmitters results in a decrease in spontaneous jerk-like movements of the extremities, trunk, face and neck (chorea) that are typical of patients with degenerative neurologic conditions such as Huntington disease. Tetrabenazine, however, does not prevent the progression or alter the outcome of these diseases. Tetrabenazine was approved by the FDA in 2008 as an orphan disease agent for treatment of the chorea associated with Huntington disease. While it is used off-label to treat other abnormal movement disorders, tetrabenazine is not formally approved for those indications. Tetrabenazine is available as tablets of 12.5 and 25 mg generically and under the brand name Xenazine. The recommended initial dose is 12.5 mg once daily, with subsequent careful increase to a maximum of 50 mg given in three divided doses daily. Because of the variable metabolism of tetrabenazine (by CYP 2D6), the maintenance dose varies by individual, and inducers or inhibitors of CYP 2D6 should be avoided. Poor CYP 2D6 metabolizers may require even higher doses. Common side effects include fatigue, sedation, somnolence, insomnia, depression, restlessness (akathisia), agitation, and nausea. Rare potentially serious adverse events include severe depression, suicidality, symptomatic hypotension, prolongation of the QTc interval and neuroleptic malignant syndrome.
Hepatotoxicity
Tetrabenazine has not been associated with rates of serum enzyme elevations greater than occur with placebo therapy, but information on liver test results during therapy is limited and occasional instances of asymptomatic ALT elevations leading to drug discontinuation or dose modification have been reported by the sponsor. In prelicensure pivotal registration trials in several hundred patients, tetrabenazine was not associated with cases of jaundice or hepatitis. Since licensure, there have been no published reports of clinically apparent liver injury, jaundice or hepatitis attributed to tetrabenazine. Thus, clinically apparent liver injury with jaundice due to tetrabenazine must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which tetrabenazine might cause liver injury is not known. Tetrabenazine undergoes hepatic metabolism largely by CYP 2D6 and is susceptible to drug-drug interactions with agents that induce or inhibit this enzyme.
Deutetrabenazine
Background
Deutetrabenazine (doo" tet ra ben' a zeen) is an inhibitor of synaptic vesicular monoamine transporter 2 (VMAT2), the inhibition of which causes a depletion of neuroactive monoamines (serotonin, norepinephrine and particularly dopamine) in nerve terminals. The reduction in these active neurotransmitters results in a decrease in spontaneous jerk-like movements of the extremities, trunk, face and neck (dyskinesia, chorea) that are typical of patients with degenerative neurologic conditions such as Huntington disease and the syndrome known as tardive dyskinesia. Tardive dyskinesia is a late ("tardive") and sometimes persistent complication of therapy with dopamine receptor anagonists including antipsychotic, antidepressant and antiemetic medications. The movements can involve any part of the body, but most characteristically cause facial tics and tongue rolling which can be particularly distressing and interfere with speech and eating. Deutetrabenazine was found to ameliorate the involuntary movements of tardive dyskinesia and Huntington disease but does not appear to prevent or retard their progression. Deutetrabenazine was approved by the FDA in 2017 for treatment of the movement disorders associated with tardive dyskinesia and Huntington disease. It is available as tablets of 6, 9 and 12 mg under the brand name Austedo. Deutetrabenazine is similar in chemical structure to tetrabenazine but has several molecules of deuterium instead of hydrogen, which creates a stronger bond with carbon and higher molecular weight. These features make it less suceptible to CYP 2D6 metabolism, improving its pharmacologic properties and allowing for twice daily dosing with more reliable drug exposure. The recommended initial dose is 6 mg once daily, with subsequent careful increase to a maximum of 48 mg (24 mg twice daily). Because of the hepatic metabolism of deutetrabenazine, the maintenance dose may be less in patients taking potent CYP 2D6 inhibitors. Common side effects resemble those of tetrabenazine and include fatigue, sedation, somnolence, insomnia, depression, restlessness (akathisia), agitation, and nausea. Rare potentially serious adverse events reported with tetrabenazine include severe depression, suicidality, symptomatic hypotension, prolongation of the QTc interval and neuroleptic malignant syndrome.
Hepatotoxicity
Deutetrabenazine has not been associated with rates of serum enzyme elevations greater than occur with placebo therapy, but information on liver test results during therapy is limited. In prelicensure pivotal registration trials in several hundred patients, deutetrabenazine was not associated with cases of jaundice or hepatitis. Since licensure, there have been no published reports of clinically apparent liver injury, jaundice or hepatitis attributed to deutetrabenazine. Thus, clinically apparent liver injury with jaundice due to deutetrabenazine must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which deutetrabenazine might cause liver injury is not known. Deutetrabenazine undergoes hepatic metabolism largely by CYP 2D6 and is susceptible to drug-drug interactions with agents that are strong inhibitors of enzyme.
Valbenazine
Background
Valbenazine (val ben' a zeen) is an inhibitor of synaptic vesicular monoamine transporter 2 (VMAT2), the inhibition of which causes a depletion of neuroactive monoamines (serotonin, norepinephrine and particularly dopamine) in nerve terminals. The reduction in these active neurotransmitters results in a decrease in spontaneous jerk-like movements of the extremities, trunk, face and neck (chorea) that are typical of patients with degenerative neurologic conditions such as Huntington disease and the abnormal involuntary movements of the condition known as tardive dyskinesia. Tardive dyskinesia is a late ("tardive") and sometimes persistent complication of therapy with dopamine receptor anagonists including antipsychotic, antidepressant and antiemetic medications. The movements can involve any part of the body, but most characteristically cause facial tics and tongue rolling which can be particularly distressing and interfere with speech and eating. Valbenazine has been shown to decrease the frequency of involuntary movements in patients with tardive dyskinesia and was approved for this use in 2017. Unlike deutetrabenazine, valbenazine has not been approved for use in Huntington disease. Valbenazine is available as tablets of 40 mg under the brand name Ingrezza. The recommended initial dose is 40 mg once daily, with subsequent increase to a maximum of 80 mg daily. Valbenazine is a prodrug that is metabolized by CYP 3A4 in the liver to its active metabolite (alpha-dihydrotetrabenazine) which is subsequently metabolized by CYP 2D6. For these reasons, its use with strong inhibitors of CYP 3A4 or 2D6 should be avoided or the dose reduced accordingly. Common side effects are similar to those of tetrabenazine and include fatigue, sedation, somnolence, insomnia, depression, restlessness (akathisia), agitation, and nausea. Rare potentially serious adverse events include severe depression, suicidality, symptomatic hypotension, prolongation of the QTc interval and neuroleptic malignant syndrome.
Hepatotoxicity
In prelicensure studies, valbenazine was not been associated with rates of serum enzyme elevations greater than occurred with placebo therapy, and no instances of clinically apparent liver injury, jaundice or hepatitis was reported, although a case of "reactivation of viral hepatitis" was reported in a patient with preexisting hepatitis C virus infection. Valbenazine has had limited general clinical use, but there have been no published reports of clinically apparent liver injury with jaundice associated with its use. Thus, clinically apparent liver injury due to valbenazine must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which valbenazine might cause liver injury is not known. Valbenazine undergoes hepatic metabolism by CYP 3A4 followed by CYP 2D6 and is susceptible to drug-drug interactions with agents that induce or inhibit these enzymes.
Drug Class: Genetic Disorder Agents, Huntington Disease Agents (for Chorea)
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Deutetrabenazine – Austedo®
Tetrabenazine – Generic, Xenazine®
Valbenazine – Ingrezza®
DRUG CLASS
Genetic Disorder Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Deutetrabenazine | 1392826-25-3 | C19-H21-D6-N-O3 (USP) C19-H27-N-O3 (FDA) |
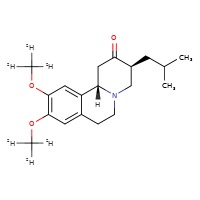 |
| Tetrabenazine | 58-46-8 | C19-H27-N-O3 |
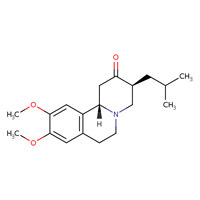 |
| Valbenazine | 1025504-45-3 | C24-H38-N2-O4 |
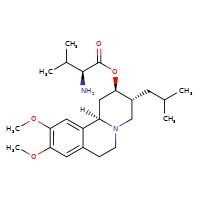 |
ANNOTATED BIBLIOGRAPHY
References updated: 02 April 2019
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 does not discuss tetrabenazine).
- Free RB, Clark J, Amara S, Sibley DR. Neurotransmission and the central nervous system. In, Brunton LL, Hilal-Danan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 243-65.(Textbook of pharmacology and therapeutics).
- Roberson ED. Huntington's disease. Treatment of central nervous system degenerative diseases. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 327-38.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy). - Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology 2006; 66: 366-72. [PubMed: 16476934](Among 84 patients with Huntington disease and chorea treated with tetrabenazine or placebo for 12 weeks, chorea scores were less with tetrabenazine, but adverse events included restlessness, agitation, depression and suicidality; 3 patients developed ALT elevations, but without symptoms or jaundice and 2 resolved without and one with dose adjustment).
- Frank S. Tetrabenazine as anti-chorea therapy in Huntington disease: an open-label continuation study. Huntington Study Group/TETRA-HD Investigators. BMC Neurol 2009; 9: 62. [PMC free article: PMC2804668] [PubMed: 20021666](Among 75 patients with Huntington disease who completed a 12 week controlled trial and were continued on tetrabenazine for up to 2 years, side effects included sedation, depression, anxiety, insomnia and akathisia; 3 patients had ALT elevations, but all resolved or improved despite continued therapy).
- Tetrabenazine (Xenazine) for Huntington's chorea. Med Lett Drugs Ther 2009; 51 (1304): 7-8. [PubMed: 19172140](Concise summary of the mechanism of action, clinical efficacy, side effects and costs of tetrabenazine, a drug available for several decades that had been recently approved for use in the US, mentions side effects of depression and suicidality, but does not mention ALT elevations or hepatotoxicity).
- Frank S. Tetrabenazine: the first approved drug for the treatment of chorea in US patients with Huntington disease. Neuropsychiatr Dis Treat 2010; 6 :657-65. [PMC free article: PMC2951749] [PubMed: 20957126](Summary of the clinical features and course of Huntington disease and its management including use of the recently approved tetrabenazine; no discussion of hepatic adverse events).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none to tetrabenazine).
- Leung JG, Breden EL. Tetrabenazine for the treatment of tardive dyskinesia. Ann Pharmacother 2011; 45: 525-31. [PubMed: 21487088](Systematic review of the literature on use of tetrabenazine for the chorea of tardive dyskinesia; mentions side effects of drowsiness, Parkinsonism and depression, but does not mention ALT elevations or hepatotoxicity).
- Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther 2012; 34: 1487-504. [PubMed: 22749259](Review of the mechanism of action, clinical efficacy and safety of tetrabenazine for treatment of hyperkinetic disorders; mentions that it is "well tolerated, although fatalities and severe reactions have been reported"; no mention of ALT elevations or hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to tetrabenazine).
- Shen V, Clarence-Smith K, Hunter C, Jankovic J. Safety and Efficacy of Tetrabenazine and Use of concomitant medications during long-term, open-label treatment of chorea associated with Huntington's and other diseases. Tremor Other Hyperkinet Mov (N Y) 2013; 3. [PMC free article: PMC3822048] [PubMed: 24255799](Among 98 patients with Huntington disease and 47 with other conditions and chorea who were treated with tetrabenazine [12.5 to 300 mg daily] for up to 11 years, 75% had a very good response at the optimal dose, and side effects included somnolence [45%], insomnia [28%], depression [27%], weight loss [14%], restlessness [12%], anxiety [10%], diarrhea and nausea [9%]; no mention of ALT elevations).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to tetrabenazine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury seen over a ten year period at 8 US medical centers, none were attributed to tetrabenazine).
- Huntington Study Group. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA 2016; 316: 40-50. [PubMed: 27380342](Among 90 adults with Huntington disease and chorea treated with deutetrabenazine [tetrabenazine with deuterium which decreases CYP 2D6 activity and prolongs its half-life] or placebo for 12 weeks, chorea was improved with the active drug and changes in scores for side effects [depression, somnolence] and laboratory values were similar in the two groups).
- Geschwind MD, Paras N. Deutetrabenazine for treatment of chorea in Huntington Disease. JAMA 2016; 316: 33-5. [PubMed: 27380339](Editorial in response to Huntington Study Group trial [2016] mentions that the degree of improvement in chorea was similar to that previously reported for tetrabenazine, but deutetrabenazine appeared to have fewer side effects).
- Kim ES. Valbenazine: first global approval. Drugs 2017; 77: 1123-9. [PubMed: 28578484](Review of the mechanism of action, pharmacology, clinical efficacy and safety of valbenazine shortly after its approval for use in tardive dyskinesia).
- Valbenazine (Ingrezza) for tardive dyskinesia. Med Lett Drugs Ther 2017; 59 (1521): 83-4. [PubMed: 28520698](Concise review of the mechanism of action, clinical efficacy, safety and costs of valbenazine shortly after its approval for tardive dyskinesia in the US discusses common side effects and drug-drug interactions, but does not mention ALT elevations or hepatotoxicity).
- Hauser RA, Factor SA, Marder SR, Knesevich MA, Ramirez PM, Jimenez R, Burke J et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry 2017; 174: 476-84. [PubMed: 28320223](Among 234 adults with tardive dyskinesia treated with valbenazine [40 or 80 mg] or placebo daily for 6 weeks, abnormal involuntary movement scores improved with valbenazine but not placebo and adverse event rates were similar although among valbenazine treated subjects, one died suddenly, one had a suicide attempt and one had “reactivation of viral hepatitis”, although the authors state that there were “no clinically relevant changes from baseline” in key laboratory parameters).
- Davis MC, Miller BJ, Kalsi JK, Birkner T, Mathis MV. Efficient trial design - FDA approval of valbenazine for tardive dyskinesia. N Engl J Med 2017; 376: 2503-6. [PubMed: 28489481](Commentary by FDA on design and facilitation of studies that led to rapid approval of valbenazine for tardive dyskinesia).
- Factor SA, Remington G, Comella CL, Correll CU, Burke J, Jimenez R, Liang GS, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 Extension Study. J Clin Psychiatry 2017; 78: 1344-50. [PubMed: 29141124](In a 42 week extension study of valbenazine for tardive dyskinesia, 198 patients were treated for up to 48 weeks of whom 63% developed an adverse event which was serious in 15%, led to discontinuation in 16% [somnolence and suicidal ideation], and death in one [due to cardiac and hepatic failure which was assessed as not related to valbenazine]).
- Fernandez HH, Factor SA, Hauser RA, Jimenez-Shahed J, Ondo WG, Jarskog LF, Meltzer HY, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: The ARM-TD study. Neurology 2017; 88: 2003-10. [PMC free article: PMC5440239] [PubMed: 28446646](Among 117 patients with tardive dyskinesia treated with tetrabenazine or placebo, abnormal involuntary movement scores improved more with tetrabenazine, but overall treatment success was similar as were total [48% vs 36%] and serious adverse event [5% vs 8%] rates; no mention of ALT elevations or hepatotoxicity).
- Heo YA, Scott LJ. Deutetrabenazine: a review in chorea associated with Huntington's disease. Drugs 2017; 77: 1857-64. [PubMed: 29080203](Review of the pharmacology, clinical efficacy and safety of deutetrabenazine mentions that adverse event rates more frequent with deutetrabenazine than placebo were somnolence [11% vs 4%], dry mouth [9% vs 7%], diarrhea [9% vs 0%], insomnia [7% vs 4% and fatigue [7% vs 4%]; no mention of ALT elevations or hepatotoxicity).
- Anderson KE, Stamler D, Davis MD, Factor SA, Hauser RA, Isojärvi J, Jarskog LF, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry 2017; 4: 595-604. [PubMed: 28668671](Among 298 patients with tardive dyskinesia treated with deutetrabenazine [12, 24 or 36 mg] or placebo daily, symptom improvements were more frequent with the two higher doses while both total and severe adverse event rates were similar in all groups; no mention of ALT elevations or hepatotoxicity).
- Frank S, Stamler D, Kayson E, Claassen DO, Colcher A, Davis C, Duker A, et al.; Huntington Study Group/Alternatives for Reducing Chorea in Huntington Disease Investigators. Safety of converting from tetrabenazine to deutetrabenazine for the treatment of chorea. JAMA Neurol 2017; 74: 977-82. [PMC free article: PMC5710322] [PubMed: 28692723](Among 37 patients with Huntington disease who were switched from tetrabenazine to deutetrabenazine at a similar dose, there was no worsening of chorea, no withdrawal because of adverse events, and “no clinically significant differences in laboratory values”).
- Niemann N, Jankovic J. Treatment of tardive dyskinesia: a general overview with focus on the vesicular monoamine transporter 2 inhibitors. Drugs 2018; 78: 525-41. [PubMed: 29484607](Review of the definition, clinical features, pathogenesis, epidemiology and management of tardive dyskinesia focusing upon the role, clinical efficacy and safety of VMAT2 inhibitors; no mention of ALT elevations or hepatotoxicity).
- Scorr LM, Factor SA. VMAT2 inhibitors for the treatment of tardive dyskinesia. J Neurol Sci 2018; 389: 43-7. [PubMed: 29433808](Review of VMAT2 inhibitors and their efficacy and safety as therapy of tardive dyskinesia concludes that they are effective and safe).
- Deutetrabenazine (Austedo) for Huntington's chorea and tardive dyskinesia. Med Lett Drugs Ther 2018; 60 (1545): 65-8. [PubMed: 29667946](Concise review of the mechanism of action, clinical efficacy, safety and costs of deutetrabenazine shortly after its approval in the US as therapy of Huntington disease and tardive dyskinesia mentions common side effects and drug-drug interactions as well as effects on QTc intervals, but does not mention ALT elevations or hepatotoxicity).
- Recent References on Deutetrabenazine: from PubMed.gov
- Trials on Deutetrabenazine: from ClinicalTrials.gov
- Recent References on Tetrabenazine: from PubMed.gov
- Trials on Tetrabenazine: from ClinicalTrials.gov
- Recent References on Valbenazine: from PubMed.gov
- Trials on Valbenazine: from ClinicalTrials.gov
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review VMAT2 inhibitors for the treatment of hyperkinetic movement disorders.[Pharmacol Ther. 2020]Review VMAT2 inhibitors for the treatment of hyperkinetic movement disorders.Koch J, Shi WX, Dashtipour K. Pharmacol Ther. 2020 Aug; 212:107580. Epub 2020 May 23.
- Review Tardive dyskinesia: placing vesicular monoamine transporter type 2 (VMAT2) inhibitors into clinical perspective.[Expert Rev Neurother. 2018]Review Tardive dyskinesia: placing vesicular monoamine transporter type 2 (VMAT2) inhibitors into clinical perspective.Citrome L. Expert Rev Neurother. 2018 Apr; 18(4):323-332. Epub 2018 Apr 2.
- Review Treatment of Tardive Dyskinesia: A General Overview with Focus on the Vesicular Monoamine Transporter 2 Inhibitors.[Drugs. 2018]Review Treatment of Tardive Dyskinesia: A General Overview with Focus on the Vesicular Monoamine Transporter 2 Inhibitors.Niemann N, Jankovic J. Drugs. 2018 Apr; 78(5):525-541.
- Review VMAT2 Inhibitors for Tardive Dyskinesia-Practice Implications.[J Pharm Pract. 2019]Review VMAT2 Inhibitors for Tardive Dyskinesia-Practice Implications.Peckham AM, Nicewonder JA. J Pharm Pract. 2019 Aug; 32(4):450-457. Epub 2018 Feb 18.
- Review Deutetrabenazine Therapy and CYP2D6 Genotype.[Medical Genetics Summaries. 2012]Review Deutetrabenazine Therapy and CYP2D6 Genotype.Dean L. Medical Genetics Summaries. 2012
- Vesicular Monoamine Transporter 2 (VMAT2) Inhibitors - LiverToxVesicular Monoamine Transporter 2 (VMAT2) Inhibitors - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...