NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Terazosin is a nonselective alpha-1 adrenergic antagonist used in the therapy of hypertension and benign prostatic hypertrophy. Terazosin therapy is associated with a low rate of transient serum aminotransferase elevations and to rare instances of clinically apparent acute liver injury.
Background
Terazosin (ter ay' zoe sin) was the second alpha-1 adrenergic antagonist to be approved for use in the United States and is still widely used for therapy of hypertension and benign prostatic hypertrophy. Terazosin inhibits alpha-adrenergic receptors present on smooth muscle in arterioles (so-called alpha-1b adrenergic receptors) as well as in those in the bladder neck and prostate (alpha-1a adrenergic receptors). The inhibition of alpha-adrenergic tone in blood vessels causes relaxation of arteriolar resistance and lowering of the blood pressure. Terazosin was approved for use in the United States in 1987 and is still used for treatment of hypertension, although rarely as a first line agent and usually in combination with other antihypertensive drugs. Terazosin is also approved for alleviation of the symptoms of urinary obstruction due to benign prostatic hypertrophy. More than 4 million prescriptions for terazosin are filled yearly. Terazosin is available in tablets or capsules of 1, 2, 5 and 10 mg generically and under the trade name Hytrin. Terazosin is usually started at a dose of 1 mg daily at bedtime with increase in the dose based upon tolerance and clinical response to an average of 5 to 10 mg daily in one dose daily, usually at bedtime. Side effects include dizziness and syncope (particularly with the initial dose), fatigue, headache, palpitations, impotence, incontinence and gastrointestinal upset. Rare, but potentially severe adverse events include postural hypotension and priapism.
Hepatotoxicity
Terazosin has been associated with a low rate of serum aminotransferase elevations that in controlled trials was no higher than with placebo therapy. These elevations were transient and did not require dose modification. Instances of serum enzyme elevations, but no instances of clinically apparent acute liver injury with jaundice due to terazosin, have been published. Furthermore, product labels do not include discussion of hepatic toxicity. Cholestatic hepatitis and jaundice have been reported with other alpha-adrenergic blockers. Thus, acute symptomatic liver injury due to terazosin must be exceedingly rare if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the minor serum aminotransferase elevations associated with terazosin is not known. Terazosin is extensively metabolized by the liver and generation of a mildly toxic intermediate is a possible explanation.
References on the safety and potential hepatotoxicity of terazosin and other alpha-blockers are given in the Overview on Alpha-1 Adrenergic Receptor Antagonists.
Drug Class: Antihypertensive Agents, Benign Prostatic Hypertrophy Agents
Other Drugs in the Subclass, Alpha-1 Adrenergic Receptor Antagonists: Doxazosin, Prazosin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Terazosin – Generic, Hytrin®
DRUG CLASS
Antihypertensive Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
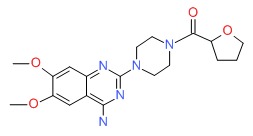
| Terazosin | 63590-64-7 | C19-H25-N5-O4 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Doxazosin.[LiverTox: Clinical and Researc...]Review Doxazosin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Alfuzosin.[LiverTox: Clinical and Researc...]Review Alfuzosin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Silodosin.[LiverTox: Clinical and Researc...]Review Silodosin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Alpha 1 Adrenergic Receptor Antagonists.[LiverTox: Clinical and Researc...]Review Alpha 1 Adrenergic Receptor Antagonists.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Tamsulosin.[LiverTox: Clinical and Researc...]Review Tamsulosin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Terazosin - LiverToxTerazosin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...