NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Telaprevir is an oral, direct acting hepatitis C virus (HCV) protease inhibitor that was used in combination with other antiviral agents in the treatment of chronic hepatitis C, genotype 1. Approved for use in the United States in 2012, it was withdrawn in 2015 when regimens of all oral direct acting agents with superior efficacy and better tolerance became available. Telaprevir was not linked to instances of acute liver injury during therapy, but was linked to cases of severe cutaneous reactions such as DRESS and Stevens Johnson syndrome which were associated with mild hepatic injury. In addition, when combined with peginterferon and ribavirin, telaprevir was associated with cases of hepatic decompensation in patients with preexisting cirrhosis.
Background
The hepatitis C virus is a small RNA virus that is a major cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma in the United States as well as worldwide. Various approaches to antiviral therapy of chronic hepatitis C have been developed, starting in the 1980s with interferon alfa which was replaced in the 1990s by long acting forms of interferon (peginterferon), to which was added the oral nucleoside analogue, ribavirin. Between 2010 and 2015, several potent oral, direct acting anti-HCV agents were developed and combinations of these found to have marked activity against the virus, allowing for highly effective therapy without use of interferon and with treatment courses of 8 to 12 weeks only. These direct acting agents included HCV protease (NS3/4) inhibitors, structural replication complex (NS5A) inhibitors and the HCV RNA polymerase (NS5B) inhibitors. The HCV proteases that have been developed are polypeptide-like molecules, modified amino acids that that resemble the specific amino acid sequence that the viral protease cleaves and act as competitive inhibitors of the protease enzyme. The first HCV protease inhibitors (all having the suffix: -previrs) approved for use in the United States were boceprevir [2012: Victrelis] and telaprevir [2012: Incevek], both used in combination with peginterferon and ribavirin. Subsequently, five more HCV protease inhibitors were approved for use in the United States: simeprevir [2013, Olysio], paritaprevir [2014, Viekira Pak], grazoprevir [2016, Zepatier], glecaprevir [2017: Mavyret], and Voxilaprevir [2017: Vosevi].
Telaprevir (tel a' pre vir), like other HCV protease inhibitors, blocks the activity of the viral encoded protease (HCV nonstructural [NS] region 3/4) that is essential in the posttranslational modification of the viral polypeptide that is cleaved into a series of structural and nonstructural (enzyme) regions. When used by itself, telaprevir results in rapid inhibition of HCV RNA levels, but resistance develops rapidly in a high proportion of patients. When combined with peginterferon and ribavirin, it was shown to provide a sustained inhibition of HCV RNA with a low rate of antiviral resistance. Triple therapy with telaprevir, peginterferon and ribavirin, when given for 24 to 48 weeks, increased the sustained virological response (SVR) rate from 40% to 50% (peginterferon and ribavirin alone) to 70% to 85% in patients with genotype 1. Telaprevir was approved for use in the United States in 2011 for patients with chronic hepatitis C, genotype 1, to be used in combination with peginterferon and ribavirin. Since that time, telaprevir has been replaced by more potent and better tolerated oral antiviral agents that can be given in combination without peginterferon. For these reasons, telaprevir was withdrawn by the sponsor in 2015. Telaprevir was previously available under the brand name Incivek (formerly VX950) as tablets of 375 mg. The recommended dose was 750 mg three times daily for the first 12 of the 24 or 48 weeks of combination therapy. The side effects of telaprevir were difficult to separate from those of peginterferon and ribavirin, but the triple therapy was associated with a higher rate of many side effects, including anemia, fatigue, itching, rash, anal pruritus and burning and gastrointestinal upset. Rash was particularly common with telaprevir therapy occurring in at least half of patients and occasionally being associated with DRESS or Stevens Johnson syndrome.
Hepatotoxicity
In large randomized controlled trials, triple therapy with telaprevir, peginterferon and ribavirin was associated with a high rate of adverse events that often required dose adjustments and led to early discontinuation in 5% to 20% of patients. However, serum ALT elevations and clinically apparent liver injury were not generally mentioned as adverse events of therapy. Telaprevir, however, was associated with a high rate of rash, which was sometimes associated with features of hypersensitivity, including rare instances of DRESS and Stevens Johnson syndrome. These severe cutaneous reactions are often accompanied by laboratory evidence of hepatic injury (ALT and alkaline phosphatase elevations). In reported cases, however, the rash and other features of hypersensitivity typically overshadowed the hepatic injury and none were reported to be associated with jaundice.
Another rare but severe hepatic complications of telaprevir therapy occurs in patients with advanced fibrosis or cirrhosis, among whom de novo, seemingly spontaneous hepatic decompensation occurred in a proportion of treated subjects. Decompensation was particularly common in patients with advanced fibrosis or cirrhosis with a previous history of decompensation. The cause of the decompensation was not clear and the separate role of telaprevir in contrast to peginterferon and ribavirin could not be defined. Nevertheless, in postmarketing studies of triple therapy of chronic hepatitis C with cirrhosis, decompensation was reported in 2% to 8% of patients, and deaths from hepatic failure in 1% to 3%.
Likelihood score for the combination of telaprevir, peginterferon and ribavirin: B (likely cause of liver injury and hepatic decompensation in patients with preexisting cirrhosis or advanced fibrosis).
Mechanism of Injury
The mechanism by which telaprevir might cause liver injury is not known. It is metabolized in the liver largely via the cytochrome P450 system, predominantly CYP 3A4, and liver injury may be due to production of a toxic or immunogenic metabolite. It is also a substrate of P-glycoprotein (P-gp). Telaprevir is also susceptible to multiple drug-drug interactions and can cause increases in serum concentrations of drugs that depend upon CYP 3A4 metabolism or P-gp. The other adverse effects of telaprevir, particularly when combined with peginterferon and ribavirin, may predispose to events that might lead to hepatic decompensation in a susceptible patient. Triple therapy using telaprevir (as well as with boceprevir and simeprevir) can cause anemia, neutropenia, thrombocytopenia, severe infections, gastrointestinal upset, dehydration and rash, all of which might help precipitate hepatic decompensation in a patient with underlying cirrhosis or advanced fibrosis.
Outcome and Management
Rash was common with telaprevir therapy and several cutaneous reactions were occasionally accompanied by evidence of hepatic injury. There is no reason to suspect cross sensitivity to the cutaneous hypersensitivity between telaprevir and other oral antivirals active against hepatitis C. Triple therapy using telaprevir is no longer used, but it was considered inadvisable in patients with preexisting cirrhosis, particularly those with a prior history of hepatic decompensation. A similar high rate of decompensation of preexisting cirrhosis was reported with triple therapy using boceprevir and simeprevir, two other HCV protease inhibitors. In fact, hepatic decompensation was also a reported complication of all-oral antiviral therapy of hepatitis C, although the rates reported with non-interferon and non-ribavirin containing regimens were quite low (<1%).
Drug Class: Antiviral Agents, Hepatitis C Agents, HCV Protease Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Telaprevir – Incivek®
DRUG CLASS
Hepatitis C Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Telaprevir | 402957-28-2 | C36-H53-N7-O6 |
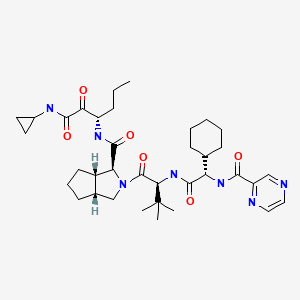
|
ANNOTATED BIBLIOGRAPHY
References updated: 26 January 2022
Abbreviation used: HCV, hepatitis C virus; HIV, human immunodeficiency virus; SVR, sustained virological response.
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013 does not discuss oral, direct acting antiviral agents used to treat hepatitis C).
- Kim JL, Morgenstern KA, Lin C, Fox T, Dwyer MD, Landro JA, Chambers SP, et al. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996;87:343–55. [PubMed: 8861917](Report of the crystal structure of the NS3/4 region of HCV with detailed description of the active serine protease catalytic site, the target for subsequent development of specific inhibitors of the HCV protease).
- Lawitz E, Rodriguez-Torres M, Muir AJ, Kieffer TL, McNair L, Khunvichai A, McHutchison JG. Antiviral effects and safety of telaprevir, peginterferon alfa-2a, and ribavirin for 28 days in hepatitis C patients. J Hepatol. 2008;49:163–9. [PubMed: 18486984](Among 12 patients treated with telaprevir, peginterferon and ribavirin for 4 weeks, all became HCV RNA negative by 28 days, and all 8 patients who continued peginterferon and ribavirin thereafter for up to 48 weeks had an SVR; during telaprevir therapy, 4 patients developed rash; no mention of de novo ALT elevations).
- Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, et al. PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–50. [PubMed: 19403903](Among 334 patients with previously untreated chronic hepatitis C, genotype 1, who received various regimens of peginterferon, ribavirin and telaprevir, SVR rates were higher with telaprevir, but were also increased by ribavirin; common adverse events included rash and pruritus; no mention of hepatic complications or de novo ALT elevations).
- McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, et al. PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38. [PubMed: 19403902](Among 240 patients with chronic hepatitis C, genotype 1, who received various regimens of telaprevir, peginterferon and ribavirin, SVR rates were higher with telaprevir as were adverse events of rash, pruritus, nausea and diarrhea, severe rash occurring in 5-9% of patients; no mention of ALT elevations or hepatic adverse events).
- McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, et al. PROVE3 Study Team. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–303. Erratum in N Engl J Med. 2010;362(17):1647. [PubMed: 20375406](Among 453 patients with previously treated chronic hepatitis C, genotype 1, treated with one of 4 regimens of peginterferon, ribavirin and telaprevir, SVR rates were higher with telaprevir [24-51% vs 14%] as were serious adverse events [17-24% vs 11%], rash occurring in at least half of patients on telaprevir, usually within 1-4 weeks of starting and requiring discontinuation in 5%; no mention of ALT elevations or liver related adverse events).
- Montaudié H, Passeron T, Cardot-Leccia N, Sebbag N, Lacour JP. Drug rash with eosinophilia and systemic symptoms due to telaprevir. Dermatology. 2010;221:303–5. [PubMed: 20798484](57 year old woman with chronic hepatitis C developed rash, fever, fatigue and lymphadenopathy 6 weeks after starting telaprevir with peginterferon and ribavirin [ALT 61 U/L, eosinophils 2,700/µL, HCV RNA negative, bilirubin and Alk P not given], resolving with prednisone therapy, but with subsequent HCV relapse; had previously received peginterferon and ribavirin without rash).
- Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, et al. ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–24. [PMC free article: PMC3809077] [PubMed: 21916639](Among 540 previously untreated patients with chronic hepatitis C, genotype 1, treated with telaprevir [12 weeks] and ribavirin and peginterferon [for 12, 24 or 48 weeks], the overall rate of SVR was 72%, rash 37% and any serious adverse events 9%; no mention of ALT elevations or liver related adverse events).
- Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, et al. REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–28. [PubMed: 21696308](Among 663 patients with previously treated chronic hepatitis C, genotype 1, who were treated with various regimens of telaprevir [12 weeks] with or without 48 weeks of peginterferon and ribavirin, SVR rates were higher with telaprevir [64-66% vs 17%] as were side effects of fatigue, gastrointestinal upset, pruritus, rash, anemia and neutropenia; no mention of liver related adverse events).
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, et al. ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. [PubMed: 21696307](Among 1088 patients with previously untreated chronic hepatitis C, genotype 1, who were treated with peginterferon and ribavirin with vs without 12 weeks of telaprevir, SVR rates were higher with telaprevir [69-75% vs 44%], but serious adverse events rates were similar [9% vs 7%]; one case of Stevens Johnson syndrome and one liver disease death occurred in telaprevir groups, but few details were provided).
- Telaprevir (Incivek) and boceprevir (Victrelis) for chronic hepatitis C. Med Lett Drugs Ther. 2011;53:57–9. [PubMed: 21778964](Concise review of the efficacy, safety and costs of boceprevir and telaprevir, shortly after their approval for use as a part of triple therapy of chronic hepatitis C, genotype 1, in the US, mentions side effects of rash, anemia, fatigue, pruritus, nausea and anorectal pruritus and burning, but not ALT elevations or clinically apparent liver injury).
- Cacoub P, Bourlière M, Lübbe J, Dupin N, Buggisch P, Dusheiko G, Hézode C, et al. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol. 2012;56:455–63. [PubMed: 21884670](Review of the clinical features and management of dermatologic side effects of triple therapy with telaprevir, during which rash arises in half of patients, usually during the first 1-12 weeks, typically mild-to-moderate in severity, but requiring discontinuation in 6% of patients, and more severe examples reported have include suspected Stevens Johnson syndrome in 3 and DRESS in 11 patients).
- Hayashi N, Okanoue T, Tsubouchi H, Toyota J, Chayama K, Kumada H. Efficacy and safety of telaprevir, a new protease inhibitor, for difficult-to-treat patients with genotype 1 chronic hepatitis C. J Viral Hepat. 2012;19:e134–42. [PMC free article: PMC3489056] [PubMed: 22239511](Among 141 previously treated Japanese patients with chronic hepatitis C, genotype 1, treated with telaprevir [12 weeks] with ribavirin and peginterferon [24 weeks], the overall SVR rate was 76% and serious adverse event rate 11%, most troublesome being anemia and rash; no mention of ALT elevations or hepatotoxicity).
- Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, Gharakhanian S, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86–96. [PubMed: 23685940](Among 62 patients with chronic hepatitis C, genotype 1, and HIV coinfection treated with telaprevir or placebo [12 weeks] and peginterferon and ribavirin [48 weeks], SVR rates were higher with telaprevir [74% vs 45%] as were serious adverse events [5% vs 0 in first 12 weeks]; no mention of ALT elevations or hepatic adverse events).
- Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, et al. CUPIC Study Group. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434–41. [PubMed: 23669289](Among 497 patients with chronic hepatitis C, genotype 1, and cirrhosis treated in a French early access program with 48 weeks of peginterferon and ribavirin with either boceprevir or telaprevir, serious adverse events occurred in 197 patients [40%], hepatic decompensation in 12 [2.4%], severe infection in 24 [4.8%], and 6 patients died [1.5%], the serious complications typically arising in the first 12 weeks of therapy).
- Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S, Pageaux GP, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60:78–86. [PubMed: 23994384](Among 37 patients with severe recurrent chronic hepatitis C, genotype 1, after liver transplantation who were treated with peginterferon, ribavirin and either boceprevir or telaprevir for up to 48 weeks, 6 [16%] had an SVR, 10 [27%] a severe infection and 3 [8%] died).
- Park C, Jiang S, Lawson KA. Efficacy and safety of telaprevir and boceprevir in patients with hepatitis C genotype 1: a meta-analysis. J Clin Pharm Ther. 2014;39:14–24. [PubMed: 24237070](Analysis of efficacy and safety of telaprevir and boceprevir in triple therapy in 10 controlled trials of 4421 patients with chronic hepatitis C, genotype 1; serious adverse events were higher with triple therapy than with peginterferon and ribavirin alone).
- Colombo M, Fernández I, Abdurakhmanov D, Ferreira PA, Strasser SI, Urbanek P, Moreno C, et al. Safety and on-treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C. Gut. 2014;63:1150–8. [PMC free article: PMC4078754] [PubMed: 24201995](Among 1587 patients with chronic hepatitis C, genotype 1, and cirrhosis treated with telaprevir [12 weeks] combined with peginterferon and ribavirin [for 24 or 48 weeks], serious adverse events were common during the first 16 weeks leading to drug discontinuation in 12% of patients and death in 7 patients; rash developed in 13% of patients, led to drug discontinuation in 5% and was compatible with Stevens Johnson syndrome in one).
- Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, De Ledinghen V, et al. CUPIC Study Group. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132–142.e4. [PubMed: 24704719](Among 511 patients with cirrhosis and chronic hepatitis C, genotype 1, and cirrhosis treated with triple therapy using telaprevir or boceprevir for 48 weeks, 91 [18%] patients had an SVR, severe adverse events occurred in 50%, including liver decompensation in 43 [8%], severe infections in 28 [5.5%] and death in 11 [2.2%]).
- Burton JR Jr, O'Leary JG, Verna EC, Saxena V, Dodge JL, Stravitz RT, Levitsky J, et al. A US multicenter study of hepatitis C treatment of liver transplant recipients with protease-inhibitor triple therapy. J Hepatol. 2014;61:508–14. [PMC free article: PMC4394742] [PubMed: 24801415](Among 81 patients with recurrent hepatitis C, genotype 1, after liver transplant who were treated with triple therapy using peginterferon, ribavirin and either boceprevir or telaprevir, the overall SVR rate was 63%, the serious adverse event rate was not reported, but 27% required hospitalization, 15% early drug discontinuation and 7 patients [9%] died of liver failure).
- Gordon SC, Muir AJ, Lim JK, Pearlman B, Argo CK, Ramani A, Maliakkal B, et al. HCV-TARGET study group. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J Hepatol. 2015;62:286–93. [PMC free article: PMC4586075] [PubMed: 25218788](Among 2084 patients with chronic hepatitis C, genotype 1, treated in clinical practice with peginterferon, ribavirin and either boceprevir or telaprevir for up to 48 weeks, the overall SVR rate was 52%, serious adverse event rate 12% while hepatic decompensation occurred in 3% and 5 patients [0.25%] died, all from hepatic failure; rash was a common side effect [63% with telaprevir and 34% with boceprevir] and was graded as serious in 8 patients [0.5%], 2 [0.1%] with DRESS).
- Miailhes P, Gilbert C, Lacombe K, Arends JE, Puoti M, Rockstroh JK, Sogni P, et al. ESCMID European Study Group on Viral Hepatitis. Triple therapy with boceprevir or telaprevir in a European cohort of cirrhotic HIV/HCV genotype 1-coinfected patients. Liver Int. 2015;35:2090–9. [PubMed: 25650873](Among 59 patients with chronic hepatitis C, cirrhosis and HIV-coinfection treated with peginterferon, ribavirin and either boceprevir [n=12] or telaprevir [n=47], the SVR rate was 36% vs 57%, and adverse events included rash [46% vs 8%] and hepatic decompensation [1 each]).
- Verna EC, Saxena V, Burton JR Jr, O'Leary JG, Dodge JL, Stravitz RT, Levitsky J, et al. CRUSH-C Consortium. Telaprevir- and boceprevir-based triple therapy for hepatitis C in liver transplant recipients with advanced recurrent disease: a multicenter study. Transplantation. 2015;99:1644–51. [PMC free article: PMC4818984] [PubMed: 25715116](Among 54 patients with advanced, recurrent chronic hepatitis C, genotype 1, after liver transplantation, who were treated with peginterferon, ribavirin and either boceprevir or telaprevir for up to 48 weeks, the SVR rate was 50%, but hepatic decompensation arose in 24% and 6 patients [11%] died).
- Neukam K, Munteanu DI, Rivero-Juárez A, Lutz T, Fehr J, Mandorfer M, Bhagani S, et al. Boceprevir or telaprevir based triple therapy against chronic hepatitis C in HIV coinfection: real-life safety and efficacy. PLoS One. 2015;10:e0125080. [PMC free article: PMC4414348] [PubMed: 25923540](Among 159 patients with chronic hepatitis C, genotype 1, treated in health care clinics in 5 European countries with peginterferon, ribavirin and either boceprevir or telaprevir for up to 48 weeks, the overall SVR rate was 63%, while 4 patients [2.5%] developed hepatic decompensation, one of whom died to hepatic failure).
- Bailly F, Pradat P, Virlogeux V, Zoulim F. Antiviral therapy in patients with hepatitis C virus-induced cirrhosis. Dig Dis. 2015;33:613–23. [PubMed: 26159282](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis summarizing the high rate of adverse events including hepatic decompensation and death with peginterferon based regimens combined with boceprevir or telaprevir, and the more effective and better tolerated all-oral regimens).
- Ferenci P, Kozbial K, Mandorfer M, Hofer H. HCV targeting of patients with cirrhosis. J Hepatol. 2015;63:1015–22. [PubMed: 26100497](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis, suggests that genotype 1 infected patients should receive an all-oral regimen such as sofosbuvir with ledipasvir or daclatasvir or the triple combination of dasabuvir with ombitasvir and paritaprevir, the major issues being duration of therapy and the role of ribavirin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 were attributed to antiviral agents, but all were antiretroviral agents and no case was attributed to the oral direct acting agents used to treat hepatitis C).
- European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199–236. [PubMed: 25911336](Guidelines for the antiviral therapy of chronic hepatitis C from the European liver disease research and academic society).
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. [PubMed: 26111063](Guidelines for the antiviral therapy of chronic hepatitis C from the US liver and infectious diseases research and academic societies).
- Carrascosa R, Capusan TM, Llamas-Velasco M, García-Buey L, Gordillo C, Sánchez-Pérez J. High frequency of severe telaprevir-associated skin eruptions in clinical practice. Acta Derm Venereol. 2016;96:97–9. [PubMed: 26073421](Among 60 patients with chronic hepatitis C, genotype 1, treated with telaprevir, peginterferon and ribavirin, 29 developed skin eruptions usually within 5-12 weeks, one with erythema multiforme and one DRESS syndrome, 6 episodes led to early discontinuation; no mention of ALT elevations or hepatic injury).
- Salmerón J, Vinaixa C, Berenguer R, Pascasio JM, Sánchez Ruano JJ, Serra MÁ, Gila A, et al. Alhambra Spanish Study Group. Effectiveness and safety of first-generation protease inhibitors in clinical practice: Hepatitis C virus patients with advanced fibrosis. World J Gastroenterol. 2015;21:9163–74. [PMC free article: PMC4533049] [PubMed: 26290644](Among 1057 patients with chronic hepatitis C who were treated with peginterferon, ribavirin and either telaprevir or boceprevir at 38 Spanish hospitals, 635 [60%] had an SVR, and adverse events were largely hematologic; no mention of ALT elevations or hepatotoxicity).
- Lepida A, Colombo M, Fernandez I, Abdurakhmanov D, Ferreira PA, Strasser SI, Urbanek P, et al. Final results of the telaprevir access program: FibroScan values predict safety and efficacy in hepatitis C patients with advanced fibrosis or cirrhosis. PLoS One. 2015;10:e0138503.(Among 1722 patients with chronic hepatitis C, genotype 1, and advanced fibrosis who were treated with peginterferon, ribavirin and telaprevir, 1139 [64%] had an SVR and common adverse events were anemia [56%], rash [30%] and pruritus [16%]; no mention of ALT elevations or hepatotoxicity).
- Coilly A, Dumortier J, Botta-Fridlund D, Latournerie M, Leroy V, Pageaux GP, Agostini H, et al. Multicenter experience with boceprevir or telaprevir to treat hepatitis C recurrence after liver transplantation: when present becomes past, what lessons for future? PLoS One. 2015;10:e0138091. [PMC free article: PMC4578772] [PubMed: 26394142](Among 81 patients with recurrent hepatitis C after liver transplantation who were treated with peginterferon, ribavirin and either telaprevir or boceprevir, 38 [47%] had an SVR, 22 [27%] a serious adverse event, 10 [12%] acute rejection, and 4 [5%] died, largely of infectious complications; no mention of ALT elevations or hepatotoxicity).
- Janczewska E, Flisiak R, Zarebska-Michaluk D, Kozielewicz D, Berak H, Dobracka B, Librant-Suska M, et al. Effect of peginterferon or ribavirin dosing on efficacy of therapy with telaprevir in treatment-experienced patients with chronic hepatitis C and advanced liver fibrosis: a multicenter cohort study. Medicine (Baltimore). 2015;94:e1411. [PMC free article: PMC4635741] [PubMed: 26402801](Among 211 treatment experienced patients with chronic hepatitis C, genotype 1, and advanced liver disease [68% with cirrhosis] treated with peginterferon, ribavirin and telaprevir, 118 [56%] had an SVR, 31 [15%] had a serious adverse reaction, and 4 died; but no death was attributed to treatment and there was no mention of ALT elevations or hepatotoxicity).
- Kawaguchi Y, Iwane S, Kumagai T, Yanagita K, Yasutake T, Ide Y, Otsuka T, et al. Efficacy and safety of telaprevir, pegylated interferon α-2b and ribavirin triple therapy in Japanese patients infected with hepatitis C virus genotype 1b. Intern Med. 2015;54:2551–60. [PubMed: 26466688](Among 106 Japanese patients with chronic hepatitis C, genotype 1b, treated with peginterferon, ribavirin and telaprevir for 24 weeks, 93 [88%] had a SVR, but adverse events were frequent including rash [28%], anemia [76%] and 31% discontinued telaprevir early; no mention of ALT elevations or hepatotoxicity).
- Ueda Y, Ikegami T, Soyama A, Akamatsu N, Shinoda M, Ishiyama K, Honda M, et al. Simeprevir or telaprevir with peginterferon and ribavirin for recurrent hepatitis C after living donor liver transplantation: A Japanese multicenter experience. Hepatol Res. 2016;46:1285–93. [PubMed: 26899352](Among 79 patients with chronic hepatitis C, genotype 1, after liver transplantation who were treated wtih peginterferon, ribavirin and either simeprevir [n=79] or telaprevir [n=36], the SVR rates were 56% vs 69%, serious adverse event rates 11% vs 25%, and immune mediated graft dysfunction occurred in 8% vs 11%).
- Montes ML, Nelson M, Girard PM, Sasadeusz J, Horban A, Grinsztejn B, Zakharova N, et al. Telaprevir-based therapy in patients coinfected with chronic hepatitis C virus infection and HIV: INSIGHT study. J Antimicrob Chemother. 2016;71:244–50. [PubMed: 26483516](Among 162 patients with chronic hepatitis C and HIV infection treated with telaprevir, peginterferon and ribavirin, hyperbilirubinemia occurred in 37 patients which was attributable to atazanavir in 36, but there were no clinically relevant changes in other hepatic laboratory results).
- Morisco F, Masarone M, Rosato V, Camera S, Granata R, Tartaglione MT, Coppola C, et al. Impact of telaprevir in HCV patients with cirrhosis and RVR: real-life data from boceprevir or telaprevir based "triple therapy" experience in Southern Italy. Rev Recent Clin Trials. 2016;11:306–316. [PubMed: 26672601](Among 103 Italian patients with cirrhosis due to HCV, genotype 1, treated with telaprevir or boceprevir with peginterferon and ribavirin, the SVR rate was 59% and serious adverse event rate 4.6%: no hepatic adverse events requiring discontinuation).
- Kumada H, Suzuki F, Kamiya N, Orihashi M, Nakayasu Y, Yamada I. Efficacy and safety of telaprevir with pegylated interferon α-2a and ribavirin in Japanese patients. Hepatol Res. 2017;47:514–521. [PubMed: 27062488](Among 54 Japanese patients with chronic hepatitis C, genotype 1, treated with telaprevir, peginterferon and ribavirin, the SVR rate was 89% while adverse events were frequent and telaprevir was discontinued early in 23 patients, largely due to rash or anemia; no mention of ALT elevations or hepatotoxicity).
- Forns X, Didier S, Mutimer D, Fagiuoli S, Navasa M, Agarwal K, Berenguer M, et al. Efficacy of telaprevir-based therapy in stable liver transplant patients with chronic genotype 1 hepatitis C. Ann Hepatol. 2016;15:512–23. [PubMed: 27236150](Among 74 patients with recurrence of HCV infection, genotype 1, after liver transplantation who were treated with telaprevir, peginterferon and ribavirin, the SVR rate was 72%, while tacrolimus and cyclosporin levels were often increased during telaprevir therapy requiring dose adjustment; common adverse events were anemia, pruritus, anorectal discomfort and rash; no mention of hepatotoxicity or hepatic decompensation).
- Ascione A, Adinolfi LE, Amoroso P, Andriulli A, Armignacco O, Ascione T, Babudieri S., Cleo Study Group, et al. Boceprevir or telaprevir in hepatitis C virus chronic infection: The Italian real life experience. World J Hepatol. 2016;8:949–56. [PMC free article: PMC4976214] [PubMed: 27574549](Among 834 patients treated with telaprevir or boceprevir combined with peginterferon and ribavirin enrolled in a multicenter Italian database, the SVR rate was 63% but adverse events were frequent and led to early discontinuation in 15%, largely for rash [31%], anemia [23%], weakness [14%] and other reasons included ascites in 3 patients, but ALT elevations and hepatic dysfunction were not mentioned).
- Flisiak R, Shiffman M, Arenas J, Cheinquer H, Nikitin I, Dong Y, Rana K, et al. A randomized study of peginterferon lambda-1a compared to peginterferon alfa-2a in combination with ribavirin and telaprevir in patients with genotype-1 chronic hepatitis C. PLoS One. 2016;11:e0164563. [PMC free article: PMC5066958] [PubMed: 27749900](Among 617 patients with chronic hepatitis C, genotype 1, treated with peginterferon alfa or lambda combined with telaprevir and ribavirin, the SVR rate was 82% [alfa] vs 76% [lambda] and while overall adverse events were similar [97% vs 92%]; lambda therapy was associated with less cytopenia than alfa, but had a higher rate of jaundice [2.7% vs 1% ] and ALT elevations [7.3% vs 0.5%] and liver abnormalities led to dose reduction in 10%).
- Iketani R, Ide K, Yamada H, Kawasaki Y, Masaki N. The safety profile of telaprevir-based triple therapy in clinical practice: a retrospective cohort study. Biol Pharm Bull. 2017;40:687–692. [PubMed: 28179602](Among 4619 patients treated for chronic hepatitis C In a nationwide Japanese database from 2009-2015, dropout rates were higher with peginterferon and ribavirin alone than with telaprevir triple therapy [23% vs 13%]; reasons for stopping telaprevir included fatigue [26%], anorexia [23%], anemia [16%], psychoneuroses [11%], retinopathy [6.1%], but no mention of hepatic adverse events).
- Mangia A, Foster GR, Berg CP, Curescu M, Ledinghen V, Habersetzer F, Manolakopoulos S, et al. PegBase Group Investigators. Efficacy and safety profile of boceprevir-or telaprevir-based triple therapy or dual peginterferon alfa-2a or alfa-2b plus ribavirin therapy in chronic hepatitis C: the real-world PegBase observational study. Ann Gastroenterol. 2017;30:327–343. [PMC free article: PMC5411384] [PubMed: 28469364](Among 4100 patients with chronic hepatitis C, genotype 1, enrolled in an international database and treated with triple therapy of peginterferon [alfa-2a or alfa-2b], ribavirin, and either boceprevir or telaprevir, SVR rates ranged from 57% to 65% and were lower in those with cirrhosis [41% vs 66%], while serious adverse events included hepatic failure [n=4: 0.1%, 1.1% of cirrhotics], serious infections [45: 1.1%] and death [11: 0.2%]).
- Callefi LA, Villela-Nogueira CA, de Barros Tenore S, Carnaúba-Júnior D, Coelho HSM, Pinto PTA, Nabuco LC, et al. Effectiveness and safety of first-generation protease inhibitors in real-world patients with hepatitis C virus genotype 1 infection in Brazil: a multicenter study. Clinics (Sao Paulo). 2017;72:378–385. [PMC free article: PMC5463255] [PubMed: 28658438](Among 715 Brazilian patients with chronic hepatitis C treated at 15 medical centers with peginterferon, ribavirin and either boceprevir [n=158] or telaprevir [n=557], the SVR rate was 57%, those with cirrhosis having a lower SVR [47% vs 71%] and higher severe adverse event rate [51% vs 35%], which included hepatic decompensation in 18 patients [4% of total, 6.6% of cirrhotics]).
- Fernández-Crehuet P, Ruiz-Villaverde R. DRESS syndrome induced by telaprevir: a potentially fatal adverse event in chronic hepatitis C therapy. Cutis. 2018;102:E4–E6. [PubMed: 30566559](58 year old woman developed severe generalized rash 2 months after the addition of telaprevir to peginterferon and ribavirin therapy of chronic hepatitis C, with fever, eosinophilia, facial edema, mild ALT elevations [60 U/L] and skin biopsy compatible with DRESS, responding rapidly to corticosteroid therapy).
- Cengiz FP, Su O, Emiroglu N, Biyik Ozkaya D, Bahali AG, Onsun N. A rare and severe cutaneous adverse effect of telaprevir: drug rash with eosinophilia and systemic symptoms. G Ital Dermatol Venereol. 2019;154:488–491. [PubMed: 31251007](64 year old man with chronic hepatitis C developed rash, fever, facial edema, lymphadenopathy and liver test elevations [AST 36 U/L, GGT 158 U/L, bilirubin 1.8 mg/dL], responding rapidly to corticosteroid therapy).
- Baker JD, Uhrich RL, Kraemer GC, Love JE, Kraemer BC. A drug repurposing screen identifies hepatitis C antivirals as inhibitors of the SARS-CoV2 main protease. PLoS One. 2021;16:e0245962. [PMC free article: PMC7850479] [PubMed: 33524017](High throughput screening of 6070 medications for activity against SARS-CoV2 in cell culture identified 8 agents with potent activity [IC50 less than 50 µM] including telaprevir and boceprevir).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Hepatitis C (HCV) Agents.[LiverTox: Clinical and Researc...]Review Hepatitis C (HCV) Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Boceprevir.[LiverTox: Clinical and Researc...]Review Boceprevir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Telaprevir: a review of its use in the management of genotype 1 chronic hepatitis C.[Drugs. 2012]Review Telaprevir: a review of its use in the management of genotype 1 chronic hepatitis C.Perry CM. Drugs. 2012 Mar 26; 72(5):619-41.
- Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial.[Lancet Infect Dis. 2015]Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial.Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, Villamil FG, Andreone P, George J, Dammers E, et al. Lancet Infect Dis. 2015 Jan; 15(1):27-35. Epub 2014 Dec 5.
- Review Telaprevir: an oral protease inhibitor for hepatitis C virus infection.[Am J Health Syst Pharm. 2012]Review Telaprevir: an oral protease inhibitor for hepatitis C virus infection.Kim JJ, Culley CM, Mohammad RA. Am J Health Syst Pharm. 2012 Jan 1; 69(1):19-33.
- Telaprevir - LiverToxTelaprevir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...