NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Talazoparib is an orally available small molecule inhibitor of the DNA repair enzyme poly ADP-ribose polymerase (PARP) which is used as an antineoplastic agent in the treatment of selected cases of breast cancer. Talazoparib is associated with a moderate rate of serum aminotransferase elevations during therapy and is suspected to cause rare instances of clinically apparent acute liver injury.
Background
Talazoparib (tal" a zoe' pa rib) is a potent small molecule inhibitor of polyadenosine 5’-diphosphoribose (ADP-ribose) polymerase (PARP), an enzyme involved in repair of single strand breaks in DNA and which is used in the therapy of breast cancer. In normal cells, DNA repair mechanisms include base excision repair for single strand DNA breaks, for which PARP plays an important role, and homologous recombination for double-strand DNA breaks for which the tumor suppressor proteins BRCA-1 and BRCA-2 are involved. In patients with cancers associated with BRCA mutations, and particularly those with breast and ovarian cancer, the cancer cells are particularly susceptible to PARP inhibitors which cause accumulation of DNA breaks and resultant cell necrosis. In several clinical trials, talazoparib has been found improve progression free survival in patients with advanced or metastatic HER-2 negative breast cancer with BRCA mutations. It is also being evaluated as therapy of other types of solid tumors that harbor germ-line BRCA mutations. Talazoparib was approved for therapy of breast cancer (HER negative, BRCA positive) in the United States in 2018 and is available in capsules of 0.25 and 1.0 mg under the brand name Talzenna. The recommended dose is 1 mg orally once daily, continued until progressive disease or intolerable toxicity occurs. Side effects are common and can include fatigue, nausea, vomiting, diarrhea, anorexia, headache, alopecia, anemia, neutropenia and thrombocytopenia. Uncommon, but potentially severe side effects include myelodysplastic syndromes, marked myelosuppression and embryo-fetal toxicity.
Hepatotoxicity
Elevations in serum aminotransferase levels are common during talazoparib therapy occurring in 33% of patients, but rising above 5 times the upper limit of the normal range in only 1%. The elevations are generally transient and not associated with symptoms or jaundice. Furthermore, similar rates of aminotransferase elevations were reported in control, comparator arms. Talazoparib has had limited clinical use but has not been linked to instances of acute liver injury with symptoms or jaundice. Because of the limited clinical experience with using talazoparib and other PARP inhibitors, their potential for causing liver injury is not well defined.
Likelihood score: E* (unproved but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The possible cause of the liver injury due to talazoparib is not known. Talazoparib has minimal hepatic metabolism and does not inhibit any of the major cytochrome P450 (CYP) metabolizing enzymes.
Outcome and Management
Talazoparib therapy has been associated with transient serum aminotransferase elevations during therapy, but has not been linked to instances of acute liver injury with jaundice or symptoms. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to temporary discontinuation, which should be permanent if laboratory values do not improve significantly or resolve within a few weeks or if symptoms or jaundice arise.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Talazoparib – Talzenna®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
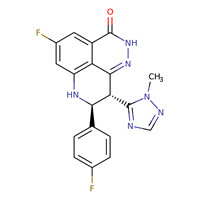
| Talazoparib | 1207456-01-6 | C19-H14-F2-N6-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 20 January 2019
Abbreviation: PARP, polyadenosine-5’-diphosphoribose polymerase.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of kinase inhibitors).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents published in 2013 before the availability of talazoparib and the small molecule PARP inhibitors).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway-targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that serum ALT elevations were frequent during talazoparib therapy, but rates were lower than with standard therapy [34% vs 39%] and there were no cases of ALT elevations with symptoms or jaundice). - de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, Kaye S, S et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov 2017; 7: 620-9. [PMC free article: PMC5905335] [PubMed: 28242752](Among 110 patients with various BRCA positive solid tumors treated with different doses of talazoparib, the maximum tolerated dose was 1.0 mg/day and extended therapy was associated with complete responses, particularly in those with ovarian and breast cancer; no mention of ALT elevations or hepatotoxicity).
- Turner NC, Telli ML, Rugo HS, Mailliez A, Ettl J, Grischke EM, Mina LA, et al. A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 Mutations (ABRAZO). Clin Cancer Res 2018 Dec 18. [Epub ahead of print] [PubMed: 30563931](Among 83 women with advanced breast cancer and BRCA mutations treated with talazoparib, the overall objective response rate was 28% and while rates of ALT elevations were not provided, two subjects stopped treatment early because of liver test abnormalities).
- Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, Fehrenbacher L, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA Mutation. N Engl J Med 2018; 379: 753-63. [PMC free article: PMC10600918] [PubMed: 30110579](Among 431 women with advanced breast cancer with BRCA mutations with talazoparib or single-agent standard therapy, median progression free survival was longer with talazoparib [8.6 vs 5.6 months], while serious adverse event rates were similar [32% vs 29%] and “hepatic toxicity” was less frequent [9% vs 20%]).
- Gunjur A. Talazoparib for BRCA-mutated advanced breast cancer. Lancet Oncol 2018; 19: e511. [PubMed: 30146245](News report on results of trial of talazoparib for breast cancer [Litton 2018] stressing the need to compare talazoparib with standard first-line platinum-based chemotherapy and to better understand mechanisms of resistance to PARP inhibitors).
- Hoy SM. Talazoparib: first global approval. Drugs 2018; 78: 1939-46. [PubMed: 30506138](Review of the mechanism of action, history of development, structure, pharmacology, efficacy and safety of talazoparib; mentions that “hepatic adverse events” were less frequent with talazoparib than standard therapy [9% vs 20%], but one patient died of suspected sinusoidal obstruction syndrome).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Talazoparib Dual-targeting on Poly (ADP-ribose) Polymerase-1 and -16 Enzymes Offers a Promising Therapeutic Strategy in Small Cell Lung Cancer Therapy: Insight from Biophysical Computations.[Cell Biochem Biophys. 2022]Talazoparib Dual-targeting on Poly (ADP-ribose) Polymerase-1 and -16 Enzymes Offers a Promising Therapeutic Strategy in Small Cell Lung Cancer Therapy: Insight from Biophysical Computations.Mgoboza C, Okunlola FO, Akawa OB, Aljoundi A, Soliman MES. Cell Biochem Biophys. 2022 Sep; 80(3):495-504. Epub 2022 May 19.
- Comparative efficacy, safety, and acceptability of single-agent poly (ADP-ribose) polymerase (PARP) inhibitors in BRCA-mutated HER2-negative metastatic or advanced breast cancer: a network meta-analysis.[Aging (Albany NY). 2020]Comparative efficacy, safety, and acceptability of single-agent poly (ADP-ribose) polymerase (PARP) inhibitors in BRCA-mutated HER2-negative metastatic or advanced breast cancer: a network meta-analysis.Wang J, Zhang Y, Yuan L, Ren L, Zhang Y, Qi X. Aging (Albany NY). 2020 Nov 30; 13(1):450-459. Epub 2020 Nov 30.
- Positron-Emission Tomographic Imaging of a Fluorine 18-Radiolabeled Poly(ADP-Ribose) Polymerase 1 Inhibitor Monitors the Therapeutic Efficacy of Talazoparib in SCLC Patient-Derived Xenografts.[J Thorac Oncol. 2019]Positron-Emission Tomographic Imaging of a Fluorine 18-Radiolabeled Poly(ADP-Ribose) Polymerase 1 Inhibitor Monitors the Therapeutic Efficacy of Talazoparib in SCLC Patient-Derived Xenografts.Laird J, Lok BH, Carney B, Kossatz S, de Stanchina E, Reiner T, Poirier JT, Rudin CM. J Thorac Oncol. 2019 Oct; 14(10):1743-1752. Epub 2019 Jun 11.
- Review Poly (ADP-Ribose) Polymerase Inhibitors: Talazoparib in Ovarian Cancer and Beyond.[Drugs R D. 2020]Review Poly (ADP-Ribose) Polymerase Inhibitors: Talazoparib in Ovarian Cancer and Beyond.Boussios S, Abson C, Moschetta M, Rassy E, Karathanasi A, Bhat T, Ghumman F, Sheriff M, Pavlidis N. Drugs R D. 2020 Jun; 20(2):55-73.
- Review A Review on Poly (ADP-ribose) Polymerase (PARP) Inhibitors and Synthetic Methodologies.[Curr Med Chem. 2021]Review A Review on Poly (ADP-ribose) Polymerase (PARP) Inhibitors and Synthetic Methodologies.Li Y, Liu CF, Rao GW. Curr Med Chem. 2021; 28(8):1565-1584.
- Talazoparib - LiverToxTalazoparib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...