NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Succimer is an oral heavy metal chelating agent used to treat lead and heavy metal poisoning. Succimer has been linked to a low rate of transient serum aminotransferase elevations during therapy, but its use has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Succimer (sux’ i mer) or dimercaptosuccinic acid (DMSA) is an orally available heavy metal chelating agent that is used to treat lead poisoning in children. Succimer is an organo-sulfur compound with two sulfhydryl groups that bind divalent metal ions such as lead, cadmium, mercury, and arsenic. Succimer does not significantly chelate essential metals such as zinc, copper or iron, and its specificity, safety and oral availability make it preferable to other chelating agents for treating lead poisoning such as Ca-EDTA which must be given intravenously and dimercaprol (British anti-Lewisite [BAL]) which requires intramuscular administration. Succimer has been shown to lower blood lead levels and improve symptoms of chronic lead poisoning. Succimer was approved for use in the United States in 1991, and current indications are for treatment of lead poisoning in pediatric patients with plasma lead levels above 45 µg/dL. It is used off label to treat adults with lead poisoning and for therapy of arsenic and mercury intoxication. Succimer is also used in naturopathic medicine administered orally as well as intravenously as a part of chelation therapy for various conditions. Succimer is available in capsules of 100 mg generically and under the brand name Chemet. The recommended dose is 10 mg/kg (or 350 mg/m2) every 8 hours for 5 days, and reduce to every 12 hours for an additional 2 weeks of therapy, with a minimum 2 week rest period between repeated courses if lead levels remain high. Side effects are generally mild and may include headache, nausea, anorexia, diarrhea, rash, neutropenia and renal dysfunction. Uncommon, but potentially severe adverse events include hypersensitivity reactions. Intravenous administration of succimer as used in naturopathic medicine has not been approved for any indication and has been associated with sudden death attributed to hypocalcemia.
Hepatotoxicity
In clinical trials conducted in children with lead poisoning, serum aminotransferase levels elevations occurred in 7% of succimer- vs 4% of placebo-treated subjects. However, ALT levels above 5 times the upper limit of normal were rare (<1% of treated patients) and no child had to stop therapy early because of liver test abnormalities. There have been no clinical reports of liver injury with jaundice attributed to succimer, but it has had limited general clinical use.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of injury accounting for serum enzyme elevations during succimer therapy is not known. Succimer-metal chelates are eliminated in the urine.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to dose reduction or temporary cessation. Succimer has not been implicated in cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. Despite the lack of evidence of significant liver injury from succimer, the product label recommends routine liver testing before and every week during therapy. There does not appear to be cross reactivity in risk for hepatic injury between succimer and other chelating agents including deferoxamine or deferiprone.
Drug Class: Chelating Agents, Lead Chelators
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Succimer – Generic, Chemet®
DRUG CLASS
Chelating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
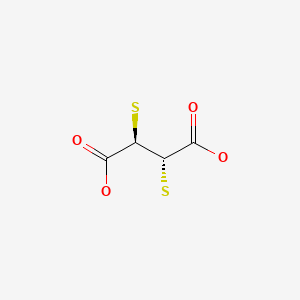
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Succimer | 304-55-2 | C4-H6-O4-S2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 August 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999; succimer is not discussed).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook on hepatotoxicity; chelating agents are not discussed).
- Byrns MC, Penning TM. Treatment of metal exposure. Environmental toxicology: carcinogens and heavy metals. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1311-15.(Textbook of pharmacology and therapeutics).
- Graziano JH, Lolacono NJ, Moulton T, Mitchell ME, Slavkovich V, Zarate C. Controlled study of meso-2,3-dimercaptosuccinic acid for the management of childhood lead intoxication. J Pediatr 1992; 120: 133-9. [PubMed: 1309865](Among 19 children with high blood lead levels [50-69 µg/dL], succimer lowered levels by 61% which was more than achieved with intravenous Ca-EDTA [45%], and repeated courses of succimer prevented the rebound that typically occurs after a single course of therapy; one child had a transient minor increase in ALT levels [26 to 62 U/L]).
- Glotzer DE. The current role of 2,3-dimercaptosuccinic acid (DMSA) in the management of childhood lead poisoning. Drug Saf 1993; 9: 85-92. [PubMed: 8397892](Review of the mechanism of action, efficacy and safety of succimer as a chelating agent for lead poisoning mentions that its safety profile is encouraging, but its use has been limited and its efficacy in preventing neurotoxicity from lead poisoning remains unproven).
- Miller AL. Dimercaptosuccinic acid (DMSA), a non-toxic, water-soluble treatment for heavy metal toxicity. Altern Med Rev 1998; 3: 199-207. [PubMed: 9630737](Review of heavy metal poisoning from lead, mercury, arsenic and cadmium and the role of succimer therapy).
- Guha Mazumder DN, Ghoshal UC, Saha J, Santra A, De BK, Chatterjee A, Dutta S, et al. Randomized placebo-controlled trial of 2,3-dimercaptosuccinic acid in therapy of chronic arsenicosis due to drinking arsenic-contaminated subsoil water. J Toxicol Clin Toxicol 1998; 36: 683-90. [PubMed: 9865236](Among 21 patients with chronic arsenic toxicity due to drinking water contamination in West Bengal, there were no differences between succimer and placebo therapy in changes in symptoms or arsenic levels after 2 two-week courses).
- Safety and efficacy of succimer in toddlers with blood lead levels of 20-44 microg/dL. Treatment of Lead-Exposed Children (TLC) Trial Group. Pediatr Res 2000; 48: 593-9. [PubMed: 11044477](Among 780 children, ages 1-3 years, with moderately elevated blood lead levels [20-44 µg/dL] treated with up to 3 courses of succimer or placebo, there was no excess of any adverse event in either group, Alk P elevations occurred in 39% of both groups, ALT elevations greater than 2 times ULN occurred in 0.8% of succimer- and 1.1% in placebo-recipients, but were transient and confirmed on repeat testing in only 1 child).
- Chisolm JJ Jr. Safety and efficacy of meso-2,3-dimercaptosuccinic acid (DMSA) in children with elevated blood lead concentrations. J Toxicol Clin Toxicol 2000; 38: 365-75. [PubMed: 10930052](Among 59 children given 116 four-week courses of succimer for lead toxicity, lead levels fell rapidly on treatment, but rebounded to 58% of pretreatment values thereafter; there were no adverse reactions).
- Centers for Disease Control and Prevention (CDC). Deaths associated with hypocalcemia from chelation therapy--Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep 2006; 55: 204-7. [PubMed: 16511441](Description of 3 cases of sudden death from hypocalcemia caused by intravenous administration of Na2-EDTA as chelation for lead poisoning or as naturopathic chelation therapy to remove heavy metals; the patient with lead poisoning was also receiving oral succimer, and Na2-EDTA was mistakenly given instead of Ca-EDTA).
- Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, Zappia I, et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: Part A--medical results. BMC Clin Pharmacol 2009; 9: 16. [PMC free article: PMC2774660] [PubMed: 19852789](Among 65 children with autism spectrum disorder, ages 3 to 8 years, treated with up to 9 three-day cycles of succimer therapy, urinary excretion of lead, tin, copper, manganese and bismuth increased significantly, and there was no significant change in any blood chemistry result).
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol 2013; 9: 347-54. [PMC free article: PMC3846971] [PubMed: 24178900](Review of the history, chemical structure, mechanism of action, preclinical and clinical assessment of the dithiol chelating agents, dimercaprol [BAL: British anti-Lewisite] and its two water soluble derivatives, dimercaptopropane sulfonic acid [DMPS, unithiol] and dimercaptosuccinic acid [DMSA: succimer] for their ability to chelate and lead to redistribution and secretion of arsenic, mercury and other heavy metals).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to succimer).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to succimer or other chelating agents).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to succimer or other chelating agents).
- Breyre A, Green-McKenzie J. Case of acute lead toxicity associated with Ayurvedic supplements. BMJ Case Rep 2016; 2016: bcr2016215041. [PMC free article: PMC4932351] [PubMed: 27364782](26 year old man developed abdominal pain and anemia after having taken Ayurvedic medications for back pain for 4 months, blood lead levels 94.8 µg/dL treated with succimer in 4 courses which lowered levels rapidly to normal, bilirubin and ALT levels were “mildly” elevated initially but resolved with control of abdominal pain before starting succimer).
- Grasso IA, Blattner MR, Short T, Downs JW. Severe systemic lead toxicity resulting from extra-articular retained shrapnel presenting as jaundice and hepatitis: a case report and review of the literature. Mil Med 2017; 182: e1843-e1848. [PubMed: 28290970](31 year old active duty military policeman developed abdominal pain, peripheral neuropathy, anemia, weakness and jaundice, having sustained severe gunshot wounds 9 years earlier with residual shrapnel in his upper thigh, who was eventually found to have elevations in blood lead levels [125 µg/mL] and who eventually improved with chelation therapy, first BAL and EDTA followed by succimer; details of liver tests not provided).
- Sakthithasan K, Lévy P, Poupon J, Garnier R. A comparative study of edetate calcium disodium and dimercaptosuccinic acid in the treatment of lead poisoning in adults. Clin Toxicol (Phila) 2018; 56: 1143-1149. [PubMed: 29889577](Among 34 adults with high blood lead levels [>40 µg/dL] treated with either succimer or EDTA in 2 five-day courses, reduction in lead levels was greater with succimer but clinical symptoms improved with both treatments, while asymptomatic ALT elevations [<3 times ULN] arose in 25% vs 14% of patients, resolving in all when therapy was stopped).
- Wilcox MA, Hardin J, Weaver J, Voss EA. Liver test monitoring: real-world compliance for drugs with monitoring requirements at 2-week intervals or more frequently. Pharmaceut Med 2019; 33: 389-94. [PubMed: 31933226](Analysis of 3 large health databases for compliance with recommendations for liver test monitoring when initiating therapy with 9 drugs found compliance in 139 patients treated with succimer to be only 29% for weekly monitoring as suggested by the product label; unclear whether succimer was being used for chelation of lead in children).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Succimer: the first approved oral lead chelator.[Am Fam Physician. 1993]Review Succimer: the first approved oral lead chelator.Jorgensen FM. Am Fam Physician. 1993 Dec; 48(8):1496-502.
- Dimercaptosuccinic Acid: Summary of Evidence.[Int J Pharm Compd. 2023]Dimercaptosuccinic Acid: Summary of Evidence.Carvin J, Thompson T. Int J Pharm Compd. 2023 Mar-Apr; 27(2):94-96.
- Review Succimer, an oral lead chelator.[Clin Pharm. 1991]Review Succimer, an oral lead chelator.Mann KV, Travers JD. Clin Pharm. 1991 Dec; 10(12):914-22.
- Review Dimercaprol.[LiverTox: Clinical and Researc...]Review Dimercaprol.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Use of orally administered succimer (meso-2,3-dimercaptosuccinic acid) for treatment of lead poisoning in dogs.[J Am Vet Med Assoc. 1996]Use of orally administered succimer (meso-2,3-dimercaptosuccinic acid) for treatment of lead poisoning in dogs.Ramsey DT, Casteel SW, Faggella AM, Chastain CB, Nunn JW, Schaeffer DJ. J Am Vet Med Assoc. 1996 Feb 1; 208(3):371-5.
- Succimer - LiverToxSuccimer - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...