NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Stiripentol is a structurally unique anticonvulsant that has been introduced as adjuvant therapy of Dravet syndrome, a rare form of severe childhood epilepsy. There is limited information on the safety of stiripentol, but it has not been associated with serum aminotransferase elevations or linked to cases of clinically apparent liver injury.
Background
Stiripentol (stir" i pen' tol) is an aromatic alcohol, structurally unrelated to other anticonvulsants that is approved for use in the United States as adjunctive therapy of seizures in patients with Dravet syndrome, a rare childhood-onset disorder marked initially by hemiclonic seizures, often triggered by fever and frequently prolonged. Myoclonic, atypical absence, focal dyscognitive and tonic seizures develop over time, and children often suffer from developmental delay and have an increased risk for sudden death during severe seizures or status epilepticus. Seizures are often refractory and problematic, usually worsening with sodium channel agents such as carbamazepine, phenytoin and lamotrigine and typically improving with clobamate and stiripentol. The anticonvulsant activity of stiripentol appears to be mediated by its ability to enhance gamma-aminobutyric acid (GABA) mediated inhibition of synaptic transmission via binding to the GABA-A receptor at a site distinct from that benzodiazepines. Stiripentol may also act by inhibition of lactic dehydrogenase, an enzyme that catalyzes the interconversion of pyruvate to lactate which results in an increase in pyruvate and ATP which secondarily affects potassium channels on neuronal cell membranes. Stiripentol was approved for use in the United States in 2018 as an anticonvulsant to be used in combination with clobazam as therapy of Dravet syndrome. Stiripentol is available in capsules of 250 and 500 mg and as a powder in 250 and 500 mg amounts for oral suspension under the brand name Diacomit. The recommended dose is 50 mg/kg daily administered in 2 or 3 divided doses. Changes in dose and discontinuations should be done gradually. Side effects are common and can include somnolence, anorexia, nausea, agitation, ataxia, hypotonia, tremor, dysarthria and insomnia. Rare, but potential severe adverse events include severe somnolence, marked weight loss, neutropenia and thrombocytopenia, and suicidal ideation and behavior.
Hepatotoxicity
Limited data are available on the safety of stiripentol, based mainly on open-label and small, placebo controlled clinical trials in children with Dravet syndrome. In these studies, addition of stiripentol to chronic clobazam therapy was not associated with an increased frequency of serum aminotransferase elevations, and there were no instances of clinically apparent liver injury. Long term therapy with stiripentol has been linked to low rates of ALT and alkaline phosphatase elevations but with increases in gamma glutamyl transpeptidase (GGT) in up to 38% of cases. Since its general availability, there have been no published case reports of stiripentol hepatotoxicity. Thus, clinically apparent liver injury due to stiripentol must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which stiripentol might cause liver injury is not known. Stiripentol is extensively metabolized by the liver via the microsomal P450 system, and it is a moderate inhibitor of CYP 2C 19 and, to a lesser extent, CYP 3A4 and 1A2. Stiripentol has major drug-drug interactions and can alter metabolism of many other anticonvulsants including clobazam which is commonly used in combination with stiripentol. Because clobazam is converted to its active metabolite by CYP 2C19 and 3A4, inhibition of the enzyme activity by stiripentol increases clobazam levels and can lead to toxicity for which reason the dose of clobazam should be reduced by half when stiripentol is added.
Outcome and Management
There is no reason to suspect cross sensitivity to hepatotoxicity of stiripentol with other drugs for epilepsy such as the benzodiazepines or aromatic anticonvulsants.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Stiripentol – Diacomit®
DRUG CLASS
Anticonvulsants
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Stiripentol | 49763-96-4 | C14-H18-O3 |
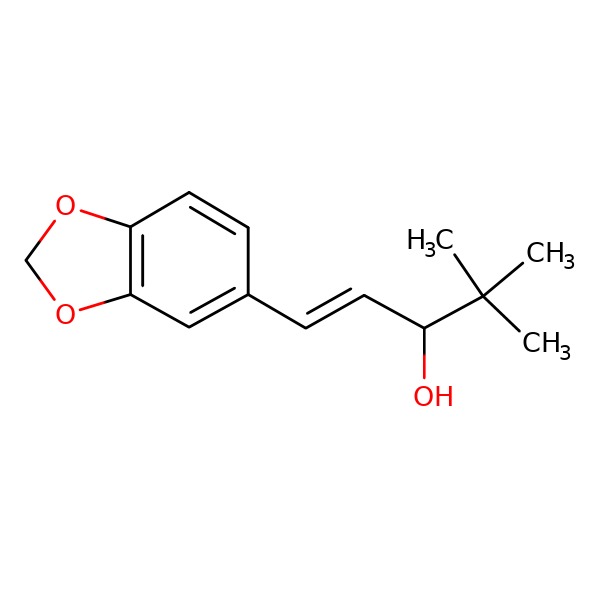 |
ANNOTATED BIBLIOGRAPHY
References updated: 10 April 2019
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; stiripentol is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; stiripentol is not discussed).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacotherapy of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that in safety analysis in several hundred treated cases there was no increase in ALT values or discontinuations for liver test abnormalities and no instances of clinically apparent liver injury). - Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia 1999; 40: 1618-26. [PubMed: 10565591](Among 212 French children with various forms of epilepsy treated with stiripentol, 48% had troublesome adverse events, most commonly neurobehavioral [30%] or gastrointestinal [29%], but only 1 child had to discontinue therapy because of adverse events; no mention of ALT elevations or hepatotoxicity).
- Chiron C, Marchand MC, Tran A, Rey E, d'Athis P, Vincent J, Dulac O, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomized placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet 2000; 356 (9242): 1638-42. [PubMed: 11089822](Among 41 children with severe myoclonic epilepsy receiving valproate and clobazam to which was added stiripentol or placebo for 2 months, clinical response rates were higher in those on stiripentol [71% vs 5%] as were adverse event rates [100% vs 40%], most responding to dose reduction and none attributed to liver injury).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to anticonvulsants).
- Wirrell EC, Laux L, Franz DN, Sullivan J, Saneto RP, Morse RP, Devinsky O, et al. Stiripentol in Dravet syndrome: results of a retrospective U.S. study. Epilepsia 2013; 54: 1595-604. [PubMed: 23848835](Survey of 13 US clinicians managing 82 children with Dravet syndrome identified adverse events in 46%, which were neurobehavioral in 27% and gastrointestinal in 10%; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none to clobazam or other benzodiazepine anticonvulsants).
- Inoue Y, Ohtsuka Y; STP-1 Study Group. Long-term safety and efficacy of stiripentol for the treatment of Dravet syndrome: A multicenter, open-label study in Japan. Epilepsy Res 2015; 113: 90-7. [PubMed: 25986195](Among 24 Japanese children started on stiripentol of whom 19 were continued for up to 40 weeks, adverse events were frequent [91%], the common events being somnolence [79%], anorexia [67%], ataxia [58%], mild ALT elevations [17%], mild Alk P [8%] and mild-to-moderate GGT [38%], but no adverse event led to drug discontinuation).
- Nickels KC, Wirrell EC. Stiripentol in the management of epilepsy. CNS Drugs 2017; 31: 405-16. [PubMed: 28434133](Review of the structure, mechanism of action, pharmacology, clinical efficacy, current uses and safety of stiripentol; mentions that adverse events can be caused by its effects on drug levels of other anticonvulsants and major problematic side effects are neurobehavioral and gastrointestinal; no mention of ALT elevations or hepatotoxicity).
- Drugs for epilepsy. Med Lett Drugs Ther 2017; 59 (1526):121-30. [PubMed: 28746301](Concise review of the currently available drugs for epilepsy does not discuss stiripentol).
- Myers KA, Lightfoot P, Patil SG, Cross JH, Scheffer IE. Stiripentol efficacy and safety in Dravet syndrome: a 12-year observational study. Dev Med Child Neurol 2018; 60: 574-8. [PubMed: 29473155](Among 41 children with Dravet syndrome followed at 2 epilepsy centers [in UK and Australia] who were treated with stiripentol for up to 12 years, 73% reported stiripentol related adverse events, most commonly anorexia, weight loss, sedation and behavioral changes and one case of protein-losing enteropathy and one of recurrent pancreatitis; ALT elevations and hepatotoxicity were not mentioned).
- Uchida Y, Terada K, Madokoro Y, Fujioka T, Mizuno M, Toyoda T, Kato D, et al. Stiripentol for the treatment of super-refractory status epilepticus with cross-sensitivity. Acta Neurol Scand 2018; 137: 432-7. [PubMed: 29313881](Among 5 adults with refractory status epilecticus and hypersensitivity reactions to various anticonvulsants [erythema multiforme, drug rash with eosinophilia and systemic symptoms, or toxic epidermal necrolysis; 3 with hepatitis], adding stiripentol led to control of seizures without recurrence of allergic reactions).
- Yıldız EP, Ozkan MU, Uzunhan TA, Bektaş G, Tatlı B, Aydınlı N, Çalışkan M, et al. Efficacy of stiripentol and the clinical outcome in Dravet syndrome. J Child Neurol 2019; 34: 33-7. [PubMed: 30362398](Among 21 Japanese children with Dravet syndrome, 86% were taking stiripentol, of whom 8 had adverse events, most commonly sedation [n=6] or ataxia [n=5], and “no patient had laboratory abnormalities in liver” function tests).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Stiripentol : in severe myoclonic epilepsy of infancy (dravet syndrome).[CNS Drugs. 2012]Review Stiripentol : in severe myoclonic epilepsy of infancy (dravet syndrome).Plosker GL. CNS Drugs. 2012 Nov; 26(11):993-1001.
- Review Stiripentol for the treatment of seizures associated with Dravet syndrome.[Expert Rev Neurother. 2019]Review Stiripentol for the treatment of seizures associated with Dravet syndrome.Chiron C. Expert Rev Neurother. 2019 Apr; 19(4):301-310. Epub 2019 Apr 2.
- Review Stiripentol for the treatment of seizures in Dravet syndrome.[Expert Rev Clin Pharmacol. 2019]Review Stiripentol for the treatment of seizures in Dravet syndrome.Eschbach K, Knupp KG. Expert Rev Clin Pharmacol. 2019 May; 12(5):379-388. Epub 2019 Apr 24.
- Stiripentol: A Novel Antiseizure Medication for the Management of Dravet Syndrome.[Ann Pharmacother. 2019]Stiripentol: A Novel Antiseizure Medication for the Management of Dravet Syndrome.Buck ML, Goodkin HP. Ann Pharmacother. 2019 Nov; 53(11):1136-1144. Epub 2019 Jun 6.
- Efficacy of Stiripentol and the Clinical Outcome in Dravet Syndrome.[J Child Neurol. 2019]Efficacy of Stiripentol and the Clinical Outcome in Dravet Syndrome.Yıldız EP, Ozkan MU, Uzunhan TA, Bektaş G, Tatlı B, Aydınlı N, Çalışkan M, Özmen M. J Child Neurol. 2019 Jan; 34(1):33-37. Epub 2018 Oct 26.
- Stiripentol - LiverToxStiripentol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...