NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Sibutramine is a serotonin and norepinephrine reuptake inhibitor which has been used for short- and long-term therapy of obesity, but which was withdrawn from use in the United States in 2010 because of increased risk of cardiovascular events. In large clinical trials, sibutramine therapy was not associated with serum enzyme elevations, and it has only rarely been implicated in cases of clinically apparent, acute liver injury.
Background
Sibutramine (si bue' tra meen) is a beta-phenylethylamine that inhibits the synaptic reuptake of both serotonin and norepinephrine. Developed initially as an antidepressant, it had little effect on depression, but its use was associated with weight loss that appeared to be due to decreased appetite and reduced caloric intake. Sibutramine was approved for use as therapy of obesity in the United States in 1997 and was widely prescribed until it was withdrawn in 2010 because of studies demonstrating an increased risk of myocardial infarction and stroke with its use. Sibutramine was previously available as capsules of 5, 10 and 15 mg under the trade name Meridia. The recommended dose was 10 mg once daily, with adjustment up to 15 mg daily or down to 5 mg daily based upon clinical effect and tolerance. Common side effects included dry mouth, headache, insomnia, constipation, nausea and increased blood pressure and heart rate. While no longer available commercially, sibutramine has been found as a contaminant in in some weight loss products available over-the-counter or via the internet.
Hepatotoxicity
Sibutramine has not been linked to an increased rate of serum enzyme elevations during therapy, but the results of serum ALT monitoring have been reported only rarely. Despite its long term availability, only a single case report of acute liver injury attributed to sibutramine has been published. The time to onset was 2 weeks and the pattern of liver enzyme elevation was cholestatic. The liver injury was anicteric and self-limited in course (Case 1). Immunoallergic and autoimmune features were absent. There have been no reports of acute liver failure or chronic liver injury attributed to sibutramine.
Mechanism of Injury
The mechanism by which sibutramine might cause liver injury is not known. Sibutramine undergoes extensive hepatic metabolism, primarily by the cytochrome P450 system (CYP 3A4) to its active metabolite which may be further metabolized and conjugated in the liver. Thus, a possible mode of liver injury is production of a toxic intermediate.
Drug Class: Weight Loss Agents
CASE REPORT
Case 1. Anicteric acute liver injury attributed to sibutramine use.(1)
A 47 year old obese woman with type 2 diabetes developed fatigue, weakness and pruritus followed by dark urine and jaundice arising 2 weeks after starting sibutramine (10 mg daily). She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. Her only other medication was insulin. She drank herbal tea. Sibutramine was taken for a total of 20 days and stopped once liver injury was identified. Laboratory testing showed normal serum bilirubin levels, but moderate elevations in ALT (222 U/L), AST (98 U/L) and alkaline phosphatase (763 U/L) (Table). Serological tests for viral hepatitis were negative as were routine autoantibodies. Abdominal ultrasound, computerized tomography and magnetic resonance imaging showed a small gallbladder polyp, but no evidence of liver enlargement or biliary obstruction. A liver biopsy showed a mixed inflammatory infiltrate without fibrosis or cholestasis. Upon stopping the medication, she began to improve clinically and laboratory tests fell rapidly and were normal when tested 8 months later.
Key Points
| Medication: | Sibutramine (10 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=0.8) |
| Severity: | 1+ (enzyme elevations without jaundice) |
| Latency: | 2 weeks to symptoms, 3 weeks to jaundice |
| Recovery: | 2-3 months |
| Other medications: | Insulin, herbal tea |
Laboratory Values
Comment
The timing of onset of liver test abnormalities, the cholestatic pattern of enzyme elevations and the liver histology were all compatible with drug induced liver injury, and the only apparent candidate was sibutramine. Normalization of the alkaline phosphatase and GGT were delayed, but this is common after cholestatic liver injury. The lack of bilirubin elevation indicates that the injury, while symptomatic, was mild.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sibutramine – Meridia®
DRUG CLASS
Weight Loss Agents
Product labeling at DailyMed, National Library of Medicine, NIH
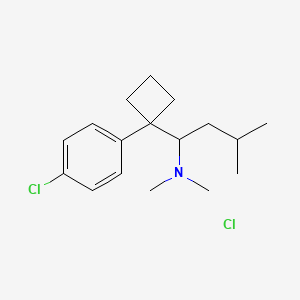
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Sibutramine | 106650-56-0 | C17-H26-Cl-N |
|
CITED REFERENCE
- 1.
- Chounta A, Tsiodras S, Zouridakis S, Doumas M, Giamarellou H. Sibutramine use associated with reversible hepatotoxicity. Ann Intern Med. 2005;143:763–4. [PubMed: 16287809]
ANNOTATED BIBLIOGRAPHY
References updated: 06 June 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-91.(Expert review of hepatotoxicity published in 1999; sibutramine is not mentioned).
- Sibley DR, Hazelwood LA, Amara SG. 5-Hydroxytryptamine (serotonin) and dopamine. In, Brunton LL, Hilal-Dandan R, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 13th ed. New York: McGraw-Hill, 2018. p. 225-42.(Textbook of pharmacology and therapeutics).
- Luque CA, Rey JA. Sibutramine: a serotonin-norepinephrine reuptake-inhibitor for the treatment of obesity. Ann Pharmacother. 1999;33:968–78. [PubMed: 10492502](Review of chemistry, pharmacology, metabolism, clinical efficacy and safety of sibutramine; no mention of hepatitis or ALT elevations in discussion of side effects).
- Bray GA. A concise review on the therapeutics of obesity. Nutrition. 2000;16:953–60. [PubMed: 11054601](Overview of the mechanism of action of antiobesity medications and their clinical efficacy and side effects; no mention of ALT elevations or liver related adverse events).
- Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord. 2002;26:262–73. [PubMed: 11850760](Metaanalysis of published studies of anti-obesity medications; sibutramine has been studied in 5 controlled trials published between 1991-98; no discussion of side effects).
- Sabuncu T, Nazligul Y, Karaoglanoglu M, Ucar E, Kilic FB. The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol. 2003;12:189–92. [PubMed: 14502318](Trial of 6 month course of sibutramine [n=13] vs orlistat [n=12] in 25 obese patients with nonalcoholic steatohepatitis; both agents were associated with weight loss and improvements in hepatic fat and ALT levels [79 to 32 U/L], but slight increase in Alk P [~175 to ~196 U/L]; no patient stopped therapy because of side effects).
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143:380–5. [PubMed: 16144896](History of the approval of medications for obesity from initial agents approved in 1947 to sibutramine in 1997; sibutramine was approved in 1997, but its long term usefulness and safety remain controversial).
- Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–46. [PubMed: 15809465](Systematic review of efficacy and safety of medications for obesity; sibutramine effects were evaluated in 29 published studies which showed an overall significant effect on weight loss when combined with lifestyle modification; no serious adverse events and no mention of hepatotoxicity).
- Chounta A, Tsiodras S, Zouridakis S, Doumas M, Giamarellou H. Sibutramine use associated with reversible hepatotoxicity. Ann Intern Med. 2005;143:763–4. [PubMed: 16287809](47 year old woman developed fatigue and pruritus 2 weeks after starting a 20 day course of sibutramine [bilirubin normal, ALT 222 U/L, Alk P 763 U/L], resolving 6-8 months after stopping: Case 1).
- Bray GA. Drug Insight: appetite suppressants. Nat Clin Pract Gastroenterol Hepatol. 2005;2:89–95. [PubMed: 16265126](Review of the mechanism of action and clinical efficacy of drugs that suppress appetite including sympathomimetic agents [amphetamine, phentermine, sibutramine], serotonin reuptake inhibitors [bupropion, fenfluramine, fluoxetine], GABAergic agents [topiramate, zonisamide], cannabinoid antagonists [rimonabant] and various peptides [leptin, neuropeptide Y, melanocortin-4]; no discussion of hepatotoxicity).
- Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006;29:277–302. [PubMed: 16569079](Review of safety of drug therapy of obesity; the only mention of liver adverse events was “a case of reversible hepatotoxicity associated with sibutramine”).
- James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, et al. SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–17. [PubMed: 20818901](Controlled trial of sibutramine vs placebo for an average of 3.4 years in 10,744 overweight or obese subjects with preexisting cardiovascular conditions, found increases in pulse rate, blood pressure, and nonfatal myocardial infarction and stokes; no mention of ALT levels or hepatotoxicity).
- Sibutramine (Meridia) withdrawn. Med Lett Drugs Ther. 2010;52(1350):88. [PubMed: 21045763](Announcement that sibutramine was withdrawn from the market because of concerns over cardiovascular safety in the US, Canada, Europe and Australia, but remains available in other countries).
- van Hunsel F, van Grootheest K. Ned Tijdschr Geneeskd. 2011;155:A3695. [Adverse drug reactions of a slimming product contaminated with sibutramine] [PubMed: 22027464](Two reports of adverse events due to "Green Coffee 800", an unregistered weight loss agent that was found to be contaminated with sibutramine; one patient had serum ALT elevations).
- Ozdemir B, Sahin I, Kapucu H, Celbis O, Karakoc Y, Erdogan S, Onal Y. How safe is the use of herbal weight-loss products sold over the Internet? Hum Exp Toxicol. 2013;32:101–6. [PubMed: 22354083](Testing of 9 weight loss products sold over the Internet found that all 9 had undeclared ingredients, 3 having sibutramine).
- Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J. 2012;36:13–25. [PMC free article: PMC3283822] [PubMed: 22363917](Review of the safety and efficacy of current and potentially future medications for obesity; mentions that the five year cardiovascular outcomes study of sibutramine [James 2010] demonstrated a significant increase in pulse, blood pressure, and risk of nonfatal myocardial infarction and stroke which led to its withdrawal in October 2010).
- Reeuwijk NM, Venhuis BJ, de Kaste D, Hoogenboom RL, Rietjens IM, Martena MJ. Active pharmaceutical ingredients detected in herbal food supplements for weight loss sampled on the Dutch market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2014;31:1783–93. [PubMed: 25247833](Sampling of 50 herbal weight loss product by the Netherlands Food and Consumer Product Safety Authority found pharmaceutically active adulterants not listed on the product label in in 24, most commonly sibutramine [n=17] but also rimonabant, sildenafil and phenolphthalein often in pharmacologically significant amounts).
- Ching CK, Chen SPL, Lee HHC, Lam YH, Ng SW, Chen ML, Tang MHY, et al. Adulteration of proprietary Chinese medicines and health products with undeclared drugs: experience of a tertiary toxicology laboratory in Hong Kong. Br J Clin Pharmacol. 2018;84:172–8. [PMC free article: PMC5736835] [PubMed: 28965348](Over a 10 year period 487 adulterated proprietary Chinese medical products were identified by a toxicology laboratory in Hong Kong, the contaminants being mostly NSAIDs, anorectics, corticosteroids and diuretics, the single most common contaminant being sibutramine [present in 155 products: 32%]).
- Dedov II, Melnichenko GA, Troshina EA, Mazurina NV, Galieva MO. Body weight reduction associated with the sibutramine treatment: overall results of the PRIMAVERA primary health care trial. Obes Facts. 2018;11:335–43. [PMC free article: PMC6189539] [PubMed: 30089303](Among 98,774 patients enrolled in a sibutramine treatment program in Russia, therapy with sibutramine resulted in weight loss in most subjects and there were no serious adverse events; no mention of ALT levels or hepatotoxicity).
- Biesterbos JWH, Sijm DTHM, van Dam R, Mol HGJ. A health risk for consumers: the presence of adulterated food supplements in the Netherlands. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2019;36:1273–88. [PubMed: 31294678](Analysis of 416 commercial dietary supplements by the Netherlands Food and Consumer Product Safety Authority between 2013 and 2018 identified 264 [64%] as having an adulterant, the most common being caffeine and synephrine, sildenafil, icariin, higenamine and sibutramine [n=22: 5.2%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Sibutramine: a serotonin-norepinephrine reuptake-inhibitor for the treatment of obesity.[Ann Pharmacother. 1999]Review Sibutramine: a serotonin-norepinephrine reuptake-inhibitor for the treatment of obesity.Luque CA, Rey JA. Ann Pharmacother. 1999 Sep; 33(9):968-78.
- [Pharmacological therapy of obesity].[G Ital Cardiol (Rome). 2008][Pharmacological therapy of obesity].Pagotto U, Vanuzzo D, Vicennati V, Pasquali R. G Ital Cardiol (Rome). 2008 Apr; 9(4 Suppl 1):83S-93S.
- Review Clinical use of sibutramine.[Drugs Today (Barc). 2004]Review Clinical use of sibutramine.Ryan DH. Drugs Today (Barc). 2004 Jan; 40(1):41-54.
- Review A benefit-risk assessment of sibutramine in the management of obesity.[Drug Saf. 2003]Review A benefit-risk assessment of sibutramine in the management of obesity.Nisoli E, Carruba MO. Drug Saf. 2003; 26(14):1027-48.
- 5-HT(1A) activation counteracts cardiovascular but not hypophagic effects of sibutramine in rats.[Obesity (Silver Spring). 2009]5-HT(1A) activation counteracts cardiovascular but not hypophagic effects of sibutramine in rats.Thomas GH, Babbs AJ, Chatfield RE, Krülle TM, Widdowson PS, Provost D, McCormack JG. Obesity (Silver Spring). 2009 Mar; 17(3):467-73. Epub 2008 Dec 11.
- Sibutramine - LiverToxSibutramine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...