NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Selinexor is a small molecule inhibitor of a nuclear exporter of proteins which is used in combination with dexamethasone in the therapy of adults with relapsed or refractory multiple myeloma. Selinexor is associated with a low rate of transient serum enzyme elevation during therapy but has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Selinexor (sel” in nex’ or) is a small molecule inhibitor of nuclear exportin-1 (XPO1) which blocks the transport of tumor suppressor proteins, oncoproteins, and growth regulators such as c-myc and cyclin D1 from the nucleus into the cytoplasm. This results in cell cycle arrest and apoptosis of cancerous but not noncancerous cells. Selinexor therapy was found to result in prolongation of progression-free survival in highly pretreated patients with advanced, relapsed or refractory multiple myeloma. Selinexor was approved for use in the United States in 2019, with indications limited to adults with relapsed or refractory multiple myeloma after failure of at least 4 previous treatment regimens. Selinexor is available tablets of 20, 40, 50 and 60 mg under the brand name Xpovio. The recommended initial dose is 80 mg on days 1 and 3 each week to continue with subsequent dose adjustment based upon tolerance. Selinexor should be used in combination with dexamethasone and with careful monitoring for side effects, platelet counts and white counts. Common side effects include neutropenia, thrombocytopenia, anemia, nausea, vomiting, anorexia, weight loss, weakness, fatigue, hyponatremia, headaches, dizziness, and confusion. Rare, but potentially serious side effects include severe hematologic toxicities including thrombocytopenia and neutropenia, severe infections, acute neurotoxicity, and embryo-fetal toxicity.
Hepatotoxicity
In prelicensure open label trials of selinexor in a total of 202 patients with advanced, refractory or relapsed multiple myeloma, serum ALT elevations arose in 8.4% of treated subjects and were above 5 times the ULN in 2.5%. The timing and character of the elevations were not described, but no patient developed raised serum enzymes with jaundice or symptoms. Since approval and general availability of selinexor, there have been no published reports of clinically apparent liver injury attributed to its use.
Likelihood score: E* (unproven, but possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the serum enzyme and bilirubin elevations that occur during selinexor therapy is unknown. Selinexor is metabolized in the liver predominantly by CYP 3A4 but does not inhibit or induce the enzyme. Its use with strong inducers of CYP 3A4 could result in decreased drug levels and reduced efficacy, while use with strong CYP 3A4 inhibitors could result in elevated drug levels and potentially increased adverse events.
Outcome and Management
The elevations in serum ALT and bilirubin during selinexor therapy have been asymptomatic, mild and transient, not requiring dose adjustments. Routine monitoring of liver tests is not recommended. There is no information on cross sensitivity for hepatic injury between selinexor and other medications used in the therapy of multiple myeloma.
Drug Class: Antineoplastic Agents, Multiple Myeloma Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Selinexor – Xpovio®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Selinexor | 1393477-72-9 | C17-H11-F6-N7O |
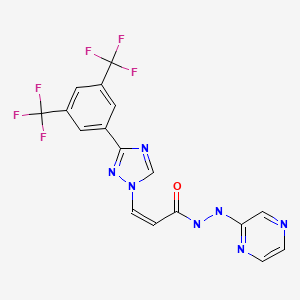
|
ANNOTATED BIBLIOGRAPHY
SELECTED BIBLIOGRAPHY
References updated: 29 April 2023
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 before the availability of selinexor).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; selinexor is not discussed).
- Isaacs C, Wellstein A, Riegel AT. Hormones and related agents in the therapy of cancer. Natural products in cancer chemotherapy: hormones and related agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1237-48.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/212306Orig1s000MultidisciplineR.pdf. (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that ALT elevations arose in 8.4% of treated patients and were above 5 times the ULN in 2.5% but no patient developed jaundice or symptoms of acute liver injury). - Grosicki S, Simonova M, Spicka I, Pour L, Kriachok I, Gavriatopoulou M, Pylypenko H, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563–1573. [PubMed: 33189178](Among 402 adults with relapsed or refractory multiple myeloma treated with bortezomib and dexamethasone with or without selinexor [100 mg once weekly], progression-free survival was longer with addition of Selinexor [13.2 vs 9.5 months]; no mention of ALT elevations or hepatotoxicity).
- Azmi AS, Uddin MH, Mohammad RM. The nuclear export protein XPO1 – from biology to targeted therapy. Nat Rev Clin Oncol. 2021;18:152–169. [PubMed: 33173198](Description of the nuclear export proteins, their role in cell integrity, and the development of specific small molecular inhibitors and their possible therapeutic activities).
- Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, Casasnovas O, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7:e511–e522. [PubMed: 32589977](Among 175 adults with relapsed or refractory diffuse large cell B cell lymphoma treated with selinexor [60 mg on days 1 and 3 weekly], the overall response rate was 28% and adverse events included thrombocytopenia, anemia, neutropenia, fatigue, and nausea, but there were no drug related deaths and no liver related severe adverse events; no mention of ALT elevations).
- Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, Moreau P, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381:727–738. [PubMed: 31433920](Among 122 adults with relapsed or refractory multiple myeloma treated with selinexor [80 mg twice weekly], 26% had at least a partial response, the median progression-free survival was 3.7 months and overall survival 8.6 months, while side effects were frequent including thrombocytopenia [73%], anemia [62%], neutropenia [40%], fatigue [73%], nausea [72%], decreased appetite [56%], weight loss [50%], diarrhea [46%], and hyponatremia [37%]; no mention of ALT elevations or hepatotoxicity).
- Syed YY. Selinexor: first global approval. Drugs. 2019;79:1485–1494. [PubMed: 31429063](Review of the mechanism of action, history of development, pharmacokinetics, clinical efficacy, and safety of Selinexor, the first-in-class, oral, small molecule inhibitor of nuclear Exportin-1; no mention of hepatotoxicity or serum ALT elevations during therapy).
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Efficacy and safety of selinexor-based regimens for relapsed/refractory multiple myeloma: a systematic review of literature.[Ann Hematol. 2022]Review Efficacy and safety of selinexor-based regimens for relapsed/refractory multiple myeloma: a systematic review of literature.Masood A, Iqbal Q, Ehsan H, Davis JA, Hansen DK, Hashmi H. Ann Hematol. 2022 Dec; 101(12):2601-2610. Epub 2022 Oct 10.
- Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma.[Blood. 2018]Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma.Bahlis NJ, Sutherland H, White D, Sebag M, Lentzsch S, Kotb R, Venner CP, Gasparetto C, Del Col A, Neri P, et al. Blood. 2018 Dec 13; 132(24):2546-2554. Epub 2018 Oct 23.
- Guidance for Use and dosing of Selinexor in Multiple Myeloma in 2021: Consensus From International Myeloma Foundation Expert Roundtable.[Clin Lymphoma Myeloma Leuk. 2022]Guidance for Use and dosing of Selinexor in Multiple Myeloma in 2021: Consensus From International Myeloma Foundation Expert Roundtable.Nooka AK, Costa LJ, Gasparetto CJ, Richardson PG, Siegel DS, Chari A, Lentzsch S, Jagannath S, Mikhael J. Clin Lymphoma Myeloma Leuk. 2022 Jul; 22(7):e526-e531. Epub 2022 Feb 4.
- Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia.[Blood. 2018]Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia.Chen C, Siegel D, Gutierrez M, Jacoby M, Hofmeister CC, Gabrail N, Baz R, Mau-Sorensen M, Berdeja JG, Savona M, et al. Blood. 2018 Feb 22; 131(8):855-863. Epub 2017 Dec 4.
- Review Clinical Utility of Selinexor/Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma: A Review of Current Evidence and Patient Selection.[Onco Targets Ther. 2020]Review Clinical Utility of Selinexor/Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma: A Review of Current Evidence and Patient Selection.Malandrakis P, Ntanasis-Stathopoulos I, Gavriatopoulou M, Terpos E. Onco Targets Ther. 2020; 13:6405-6416. Epub 2020 Jul 1.
- Selinexor - LiverToxSelinexor - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...