NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The sodium-glucose cotransporter-2 (SGLT2) inhibitors are diabetic agents that act by inhibiting the reabsorption of glucose in the proximal renal tubule, resulting in loss of glucose in the urine and reduction in serum levels. SGLT2 is the major enzyme responsible for glucose reabsorption in the kidney and its inhibition causes a reduction in the threshold for glucose loss in urine. The excess loss of glucose causes a loss of calories, reduction in serum glucose and mild osmotic diuresis. The SGLT2 inhibitors also cause a modest weight loss and slight decrease in blood pressure, both of which may contribute to their beneficial effects. Five specific SGLT2 inhibitors, bexagliflozin, canagliflozin, dapagliflozin, empagliflozin and ertugliflozin, have been shown to result in improvements in glycemic control in type 2 diabetes and introduced into clinical use. Canagliflozin, dapagliflozin and empagliflozin have also been shown to decrease cardiovascular complications and mortality in patients with type 2 diabetes and cardiovascular disease, and to reduce the risk of hospitalization for heart failure and development of end stage renal disease in patients with type 2 diabetes and chronic kidney disease. In prelicensure studies, none of the five agents was reported to be associated with increases in serum aminotransferase or alkaline phosphatase levels and, since licensure, there have been only very rare, isolated, and not completely convincing reports of clinically apparent liver injury associated with their use.
Background
Bexagliflozin (bex’ a gli floe' zin) is a specific SGLT2 inhibitor that in clinical trials was shown to result in a reduction in serum HbA1c levels and better glycemic control in type 2 diabetes, both as monotherapy (in patients who failed to achieve adequate control on diet and exercise) or in combination with insulin, metformin and sulfonylureas. Bexagliflozin was approved for use in the United States in 2023 for management of hyperglycemia in patients with type 2 diabetes in conjunction with diet and exercise, with or without other antidiabetic medications. Bexagliflozin is available in tablets of 20 mg under the brand name Brenzavvy, the recommended dose being 20 mg once daily. Common side effects shared by all SGLT2 inhibitors include symptoms of thirst, urinary tract infections and mycotic genital infections. Less common side effects are hypersensitivity reactions, ketoacidosis, hypoglycemia, dehydration, hypovolemia and serum creatinine elevations. Bexagliflozin may be associated with an increased risk of lower limb amputations and necrotizing fasciitis of the perineum in patients with type 2 diabetes.
Canagliflozin (kan" a gli floe' zin) is a specific SGLT2 inhibitor that in clinical trials was shown to result in a reduction in serum HbA1c levels and improved glycemic control in type 2 diabetes, both as monotherapy (in patients who failed to achieve adequate control on diet and exercise) or in combination with insulin, metformin and/or sulfonylureas. Canagliflozin was approved for use in the United States in 2013 and current indications are for management of hyperglycemia in patients with type 2 diabetes in conjunction with diet and exercise, with or without other antidiabetic medications. After long term controlled trials, canagliflozin was also shown to reduce the risk of major adverse cardiovascular events (myocardial infarction, stroke and death) for patients with type 2 diabetes at risk for cardiovascular disease and was later shown to reduce the risk of end stage renal disease and hospitalization of heart failure for patients with diabetic nephropathy. For these reasons, canagliflozin is now recommended for these indications as well. Canagliflozin is available in tablets of 100 and 300 mg under the brand name Invokana, the recommended dose being 100 to 300 mg once daily. Combinations of canagliflozin including extended release forms with metformin are also available (Invokamet and Invokamet XR). Common side effects shared by all SGLT2 inhibitors include symptoms of thirst, urinary tract infections and mycotic genital infections. Less common side effects are hypersensitivity reactions, bone fractures, ketoacidosis, hypoglycemia, dehydration, hypovolemia and serum creatinine elevations. Canagliflozin may also be associated with an increased risk of lower limb amputations and necrotizing fasciitis of the perineum in patients with type 2 diabetes.
Dapagliflozin (dap' a gli floe' zin) is a specific SGLT2 inhibitor that has been shown to result in a reduction in serum HbA1c levels and better control of type 2 diabetes, both as monotherapy (in patients who failed to achieve adequate glycemic control on diet and exercise) or in combination with insulin, metformin and or sulfonylureas. Dapagliflozin was approved for use in the United States in 2014 and current indications are to improve glycemic control in patients with type 2 diabetes in conjunction with diet and exercise, with or without other antidiabetic medications. Long term therapy is also recommended for reduction in major cardiovascular outcomes (myocardial infarction, stroke, death), progression of renal disease and end stage renal failure, and hospitalization and death from heart failure in patients with type 2 diabetes at risk for these complications. Dapagliflozin is available in tablets of 5 and 10 mg under the brand name Farxiga, the recommended dose being 5 to 10 mg once daily. Fixed combinations of dapagliflozin with saxagliptin are available (Qtern) as are extended release combinations with metformin (Xigduo XR). Common side effects shared by all SGLT2 inhibitors include symptoms of thirst, urinary tract infections and mycotic genital infections. Less common side effects are hypersensitivity reactions, ketoacidosis, hypoglycemia, dehydration, hypovolemia and serum cholesterol and creatinine elevations. Dapagliflozin may also be associated with an increased risk of necrotizing fasciitis of the perineum in patients with type 2 diabetes.
Empagliflozin (em" pa gli floe' zin) is a specific SGLT2 inhibitor that in clinical trials was shown to result in a reduction in serum HbA1c levels and better glycemic control in type 2 diabetes, both as monotherapy (in patients who failed to achieve adequate control on diet and exercise) or in combination with insulin, metformin and or sulfonylureas. Empagliflozin was approved for use in the United States in 2014 for management of hyperglycemia in patients with type 2 diabetes in conjunction with diet and exercise, with or without other antidiabetic medications. In 2017, the indications for empagliflozin were extended to decrease the risk of cardiovascular death in adults with type 2 diabetes and cardiovascular disease, and later to decrease the risk of hospitalization or death from heart failure in adults with type 2 diabetes with heart failure. Empagliflozin is available in tablets of 10 and 25 mg under the brand name Jardiance, the recommended initial dose being 10 mg once daily, which can be increased to 25 mg daily. Fixed dose combinations of empagliflozin with metformin (Synjardy) and with linagliptin (Glyxambi) are also available. Common side effects shared by all SGLT2 inhibitors include symptoms of thirst, urinary tract infections and mycotic genital infections. Less common side effects are hypersensitivity reactions, ketoacidosis, hypoglycemia, dehydration, hypovolemia and serum creatinine elevations. Empagliflozin may also be associated with an increased risk of necrotizing fasciitis of the perineum in patients with type 2 diabetes.
Ertugliflozin (er" too gli floe' zin) is a specific SGLT2 inhibitor that in clinical trials was shown to result in a reduction in serum HbA1c levels and better glycemic control in type 2 diabetes, both as monotherapy (in patients who failed to achieve adequate control on diet and exercise) or in combination with insulin, metformin and sulfonylureas. Ertugliflozin was approved for use in the United States in 2018 for management of hyperglycemia in patients with type 2 diabetes in conjunction with diet and exercise, with or without other antidiabetic medications. Ertugliflozin is available in tablets of 5 and 15 mg under the brand name Steglatro, the recommended initial dose being 5 mg once daily, which can be increased to 15 mg daily. Fixed dose combinations of ertugliflozin with metformin (Segluromet) and with sitagliptin (Steglujan) are also available. Common side effects shared by all SGLT2 inhibitors include symptoms of thirst, urinary tract infections and mycotic genital infections. Less common side effects are hypersensitivity reactions, ketoacidosis, hypoglycemia, dehydration, hypovolemia and serum creatinine elevations. Ertugliflozin may be associated with an increased risk of lower limb amputations and necrotizing fasciitis of the perineum in patients with type 2 diabetes.
Hepatotoxicity
In multiple large randomized controlled trials, canagliflozin, dapagliflozin, empagliflozin and ertugliflozin were not associated with serum enzyme elevations during therapy. Indeed, in retrospective analyses, therapy with SGLT2 inhibitors was associated with improvements in ALT levels, probably as a result of concurrent improvements in fatty liver disease due to improved glycemic control or weight loss or both. During prelicensure studies, no instances of clinically apparent acute liver injury were convincingly linked to use of the SGLT2 inhibitors, and serum enzyme elevations accompanied by jaundice occurred equally in the actively treated and placebo groups. Since their approval and more widespread use, at least one report of liver injury possibly due to a SGLT2 inhibitor has been published. A woman with nonalcoholic fatty liver disease and cirrhosis developed decompensation with jaundice, ascites and encephalopathy 10 weeks after starting dapagliflozin. She improved somewhat after stopping therapy, but ultimately required liver transplantation several months later. Thus, hepatotoxicity from canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and bexagliflozin is quite rare, if it occurs at all.
Bexagliflozin likelihood score: E (unlikely cause of clinically apparent liver injury).
Canagliflozin likelihood score: E (unlikely cause of clinically apparent liver injury).
Dapagliflozin likelihood score: D (possible rare cause of clinically apparent liver injury).
Empagliflozin likelihood score: E (unlikely cause of clinically apparent liver injury).
Ertugliflozin likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The relative lack of hepatotoxicity of the SGLT2 inhibitors may relate to their minimal hepatic metabolism which is largely via UDP-glucuronylsyltransferase (UGT-1A9 and 2B4 among others).
Outcome and Management
Liver injury from the SGLT2 inhibitors is rare, and they have not been associated with acute liver failure, vanishing bile duct syndrome or chronic hepatitis. The similarity of structure and function of the SGLT2 inhibitors suggests that there may be some degree of cross sensitivity to their adverse events.
Drug Class: Antidiabetic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bexagliflozin – Brenzavvy®
Canagliflozin – Invokana®
Dapagliflozin – Farxiga®
Empagliflozin – Jardiance®
Ertugliflozin – Steglatro®
DRUG CLASS
Antidiabetic Agents
COMPLETE LABELING [Canagliflozin]
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bexagliflozin | 1118567-05-7 | C24-H29-Cl-O7 |
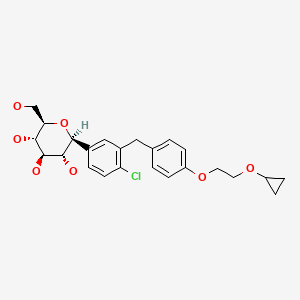
|
| Canagliflozin | 928672-86-0 | C24-H25-F-O5-S.1/2H2-O |
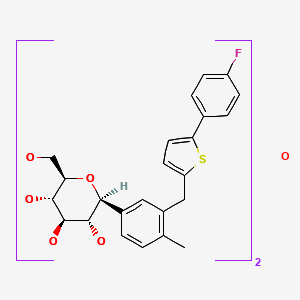
|
| Dapagliflozin | 461432-26-8 | C21-H25-Cl-O6 |
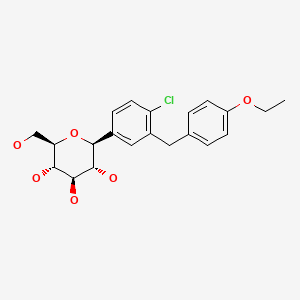
|
| Empagliflozin | 864070-44-0 | C23-H27-Cl-O7 |
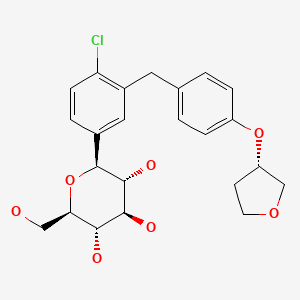
|
| Ertugliflozin | 1210344-57-2 | C22-H25-Cl-O7 |

|
ANNOTATED BIBLIOGRAPHY
References updated: 10 February 2023
Abbreviations: CAP, controlled attenuation parameter; GLP-1, glucagon-like peptide-1; HbA1c, hemoglobin A1c; SGLT2, sodium-glucose cotransporter-2.
- Zimmerman HJ. Oral hypoglycemic agents and other diabetes therapy. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 575-9.(Textbook of hepatotoxicity published in 1999 and before the availability of SGLT2 inhibitors).
- De Marzio DH, Navarro VJ. Antidiabetic drugs. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 528-32.(Review of hepatotoxicity of drugs for diabetes, does not discuss the SGLT2 inhibitors).
- Powers AC, D'Alessio D. Therapy of diabetes. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycemia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1248-67.(Textbook of pharmacology and therapeutics).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver injury in the US collected from 2004 to 2008, none were attributed SGLT2 inhibitors, which were rarely used during the period of this study).
- Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24. [PMC free article: PMC2945163] [PubMed: 20566676](Among 485 patients with type 2 diabetes who were not adequately controlled on diet and exercise advice were treated with one of 3 doses of dapagliflozin or placebo for 24 weeks, there was an increased incidence of signs and symptoms of urinary tract and genital infections on dapagliflozin: no mention of ALT levels of clinically apparent liver injury).
- Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S., Dapagliflozin 006 Study Group. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–15. [PubMed: 22431673](Among 805 patients with type 2 diabetes on insulin treated with dapagliflozin or placebo, those on dapagliflozin had higher rates of hypoglycemia episodes, genital and urinary tract infections, and weight loss [1.0-1.5 kg]; no discussion of ALT results or hepatotoxicity).
- Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140–51. [PubMed: 24622320](Review of mechanisms of action, structure, pharmacokinetics, efficacy and safety of the SGLT inhibitors; no mention or discussion of ALT elevations or hepatotoxicity).
- Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74. [PubMed: 24026259](Metaanalysis of publications on the safety and efficacy of the SGLT2 inhibitors states that: “regarding liver related adverse events, regulatory authorities’ reports concluded that slight imbalance among patients treated with dapagliflozin or canagliflozin and control groups were probably not associated with the study drug”).
- Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–15. [PMC free article: PMC3747923] [PubMed: 23564919](Among 756 patients treated with the addition of either canagliflozin or sitagliptin to stable therapy for 52 weeks, adverse events rates were similar in the two groups, except for higher rates of genital mycotic infections and small-to-moderate decreases in ALT, GGT and weight loss in the canagliflozin treated patients; no liver related serious adverse events in either group).
- Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–82. [PMC free article: PMC3593184] [PubMed: 23279307](Among 584 patients with type 2 diabetes treated with canagliflozin [200 or 300 mg] or placebo for 26 weeks, there were increased rates of urinary tract and genital infections and were modest improvements in ALT and Alk P levels).
- Canagliflozin (Invokana) for type 2 diabetes. Med Lett Drugs Ther. 2013;55(1416):37–9. [PubMed: 23669782](Concise overview on the use of canagliflozin in diabetes shortly after its approval for use in the US; mentions side effects of genital mycotic infections [10-15%], urinary tract infections [5%] and serum cholesterol elevations; no mention of hepatotoxicity or changes in serum ALT levels).
- Dapagliflozin (Farxiga) for type 2 diabetes. Med Lett Drugs Ther. 2014;56(1436):13–5. [PubMed: 24663030](Concise overview on the use of dapagliflozin in diabetes shortly after its approval for use in the US; mentions increased risk of mycotic genital infections and urinary tract infections, and its potential to cause hypovolemia and cholesterol elevations; no discussion of hepatotoxicity or serum ALT elevations).
- Drugs for type 2 diabetes. Treat Guidel Med Lett. 2014;12(139):17–24. [PubMed: 24566424](Concise overview and recommendations on the use of medications in diabetes; the SGLT2 inhibitors can reduce HbA1c levels by 0.5-1.0% and cause mild weight loss).
- Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC. EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–19. [PubMed: 24622369](Among 899 patients with diabetes treated with empagliflozin at 2 doses, or sitagliptin, or placebo for 24 weeks; empagliflozin treated patients had more weight loss and higher rates of urinary tract and mycotic genital infections; no mention of ALT levels or hepatotoxicity).
- Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, Woerle HJ. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–60. [PubMed: 23906374](Among 495 patients with diabetes inadequately controlled on metformin treated with one of 5 doses of empagliflozin or sitagliptin or placebo for 12 weeks, adverse events included genital mycotic infections, urinary tract infections and frequent urination: no mention of ALT elevations or hepatotoxicity).
- Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ., EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–9. [PubMed: 24722494](Among 638 patients with diabetes inadequately controlled on metformin treated with 2 doses of empagliflozin or placebo for 24 weeks, adverse events with empagliflozin included hypoglycemia [1-2%], urinary tract infections [5-6%] and genital infections [12%]; no mention of ALT elevations or hepatotoxicity).
- Jahagirdar V, Barnett AH. Empagliflozin for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2014;15:2429–41. [PubMed: 25301180](Review of the safety and efficacy of empagliflozin based upon 2 published phase II and 4 phase III studies concluded that “empagliflozin was well tolerated”; no mention of ALT elevations or hepatotoxicity).
- Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. EMPA-REG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. [PubMed: 24948511](1549 patients with diabetes inadequately controlled on metformin, were randomly assigned to be treated with the addition of empagliflozin or glimepiride for 2 years; improvements in HbA1c and adverse events were both similar in both groups; reasons for discontinuation of empagliflozin included one case each of hepatic failure, acute hepatitis and jaundice with no further details; no mention of change in ALT levels).
- Empagliflozin (Jardiance) for diabetes. Med Lett Drugs Ther. 2014;56(1453):99–100. [PubMed: 25296258](Concise review of the mechanism of action, efficacy, safety and cost of empagliflozin shortly after its approval for use in the US, states that adverse events include urinary tract and mycotic genital infections and dehydration; no mention of ALT elevations or hepatotoxicity).
- Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37:815–29. [PubMed: 25096959](In a pooled analysis of 8685 patients from 12 controlled trials of dapagliflozin in type 2 diabetes, rates of adverse events were 17% vs 13% with placebo, including genital infections [11% vs 1%], urinary tract infections [6% vs 4%], polyuria [3% vs 1.7%] and liver test elevations [4% vs 4%]; mean ALT values decreasing by 3.9 U/L vs 1.7 U/L while combined liver enzyme and bilirubin elevations arose in 0.1% vs 0.2%).
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. [PubMed: 26378978](Among 7020 patients with type 2 diabetes at high risk for cardiovascular disease treated with empagliflozin [10 or 25 mg] or placebo once daily for a median of 3.1 years, cardiovascular death rates were lower with the SGLT2 inhibitor therapy [3.7% vs 5.9%], while total and serious adverse event rates were similar in the 3 groups and no differences in changes from baseline of routine liver tests).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 4 cases [0.5%] were at least possibly attributed to antidiabetic drugs, but none to SGLT2 inhibitors).
- Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25–32. [PubMed: 26575250](Among 2313 patients with type 2 diabetes treated with canagliflozin or placebo for 26 weeks in 4 controlled trials, ALT, AST and Alk P levels decreased more with canagliflozin than placebo, but the decreases correlated more with reductions in HbA1c levels and weight rather than canagliflozin).
- Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, Vilsbøll T. Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2016;11:e0166125. [PMC free article: PMC5106000] [PubMed: 27835680](Systematic review of 18 controlled trials of SGLT2 inhibitors in type 2 diabetes found that SGLT2 inhibitors reduced ALT levels compared to placebo by an average of 2.8 U/L).
- Levine JA, Ann Lo A, Wallia A, Rogers M, VanWagner LB. Dapagliflozin-induced acute-on-chronic liver injury. ACG Case Rep J. 2016;3:e169. [PMC free article: PMC5171930] [PubMed: 28008402](A 48 year old woman with cirrhosis due to nonalcoholic steatohepatitis and type 2 diabetes developed jaundice and ascites 10 weeks after starting dapagliflozin [bilirubin 1.2 before treatment rising to 20 mg/dL, ALT 45 to 78 U/L, Alk P 220 to 188 U/L, INR 1.5, creatinine 0.8], improving somewhat when the drug was stopped, but qualifying for and then undergoing liver transplantation 4 months later).
- Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25–32. [PubMed: 26575250](Among 2313 patients with type 2 diabetes enrolled in 6 placebo-controlled trials of canagliflozin, serum levels of ALT, AST, GGT and Alk P decreased with canagliflozin but not placebo and the degree of improvements correlated with both weight loss and improvements in HbA1c levels, an additive effect).
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. [PubMed: 28605608](Among 10142 adults with type 2 diabetes at risk for cardiovascular disease treated with canagliflozin [100 or 300 mg] or placebo once daily for an average of 3.6 years in 2 randomized controlled trials, major cardiovascular events [death, myocardial infarction, stroke] were less with canagliflozin [26.9 vs 31.5 events per 1000 patient-years], while adverse event rates were similar except for lower extremity amputations [6.3 vs 3.4 per 1000 patient-years]; no mention of ALT elevations or hepatotoxicity).
- Famularo G, Sajeva MR, Marino G, Granato C. Acute hepatitis caused by empagliflozin in a nonalcoholic fatty liver disease patient. Ann Pharmacother. 2017;51:1142–3. [PubMed: 28845675](75 year old woman with type 2 diabetes developed fever and jaundice 6 weeks after starting empagliflozin [bilirubin 4.1 mg/dL, ALT 311 U/L, Alk P 587 U/L], which resolved within a week of stopping and remained normal thereafter, liver tests having been normal in the past despite having fatty liver by ultrasound).
- Terra SG, Focht K, Davies M, Frias J, Derosa G, Darekar A, Golm G, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19:721–8. [PubMed: 28116776](Among 461 patients with type 2 diabetes treated with ertugliflozin [5 or 15 mg] or placebo daily for 26 weeks, improvements in glycemic control and weight loss were greater with active treatment while adverse event rates were no different from placebo except for genital mycotic infections; no mention of ALT levels or hepatotoxicity).
- Miller S, Krumins T, Zhou H, Huyck S, Johnson J, Golm G, Terra SG, et al. Ertugliflozin and sitagliptin co-initiation in patients with type 2 diabetes: the VERTIS SITA randomized study. Diabetes Ther. 2018;9:253–68. [PMC free article: PMC5801244] [PubMed: 29313282](Among 291 patients with type 2 diabetes treated with the combination of ertugliflozin [5 or 15 mg] and sitagliptin [100 mg] or placebo daily, glycemic control was better and decreases in HbA1c greater with the active treatment, while adverse event rates were similar in all three groups; no mention of ALT elevations or hepatotoxicity).
- Hollander P, Liu J, Hill J, Johnson J, Jiang ZW, Golm G, Huyck S, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the VERTIS SU randomized study. Diabetes Ther. 2018;9:193–207. [PMC free article: PMC5801240] [PubMed: 29282633](Among 1326 patients with type 2 diabetes inadequately controlled on metformin treated with the addition of ertugliflozin [5 or 15 mg] or glimepiride [~3 mg] daily, HbA1c decreases were similar in the 3 groups [-0.6 to -0.7%] and adverse event rates were similar except for higher rates of genital mycotic infections in patients on ertugliflozin; no mention of ALT elevations or hepatotoxicity).
- Aronson R, Frias J, Goldman A, Darekar A, Lauring B, Terra SG. Long-term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes Metab. 2018;20:1453–60. [PMC free article: PMC5969239] [PubMed: 29419917](Extension, open label assessment of ertugliflozin in diabetic patients enrolled in a placebo-controlled trial [Terra 2017], found that the improvements in glycemic control were maintained, and while mean levels of ALT and AST decreased, one patient on 5 mg daily of ertugliflozin had ALT and AST elevations "possibly related to study medication" with no details provided).
- Pratley RE, Eldor R, Raji A, Golm G, Huyck SB, Qiu Y, Sunga S, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: The VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20:1111–20. [PMC free article: PMC5947297] [PubMed: 29266675](Among 1233 patients with type 2 diabetes inadequately controlled on metformin treated with ertugliflozin [5 or 15 mg] or sitagliptin [100 mg] or both, improvements in HbA1c and glycemic control were greatest with the combination, while adverse event rates were similar among groups except for higher rates of genital mycotic infections in those on ertugliflozin).
- Ertugliflozin for type 2 diabetes. Med Lett Drugs Ther. 2018;60(1545):70–2. [PubMed: 29667948](Concise review of the mechanism of action, clinical efficacy, safety and costs of ertugliflozin, the fourth SGLT2 inhibitor approved for use in the US; mentions side effects of genital mycotic infections and possible adverse reactions of hypovolemia, dehydration, renal injury, and increased risk for lower-limb amputations; no mention of ALT elevations or hepatotoxicity).
- Markham A. Ertugliflozin: first global approval. Drugs. 2018;78:513–9. [PubMed: 29476348](Review of the mechanism of action, chemistry, pharmacology, clinical efficacy, and safety of ertugliflozin shortly after its US approval; mentions frequency of genital mycotic infections on treatment and that non-traumatic lower limb amputations occurred in 11 of 3409 [0.3%] ertugliflozin vs 1 of 1450 [<0.1%] comparator group patients; no mention of ALT elevations or hepatotoxicity).
- Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care. 2018;41:1801–8. [PubMed: 29895557](Among 42 patients with type 2 diabetes and fatty liver treatment with empagliflozin was associated with greater decline in body weight [-3.3 vs -1.6 kg], ALT levels [-15 vs -4 U/L] and liver fat as assessed by magnetic resonance imaging [-5% vs -1%]).
- Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923–34. [PMC free article: PMC6096619] [PubMed: 29971527](Among 84 patients with type 2 diabetes and fatty liver treated with dapagliflozin or n-3 carboxylic acids or both or placebo, ALT levels, HbA1c and body weight declined with dapagliflozin therapy whereas liver fat volume was decreased in all 3 active therapy groups).
- Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61:2155–63. [PMC free article: PMC6133166] [PubMed: 30066148](Among 7020 patients with type 2 diabetes enrolled in a large, long term placebo-controlled trial of empagliflozin [Zinman 2015], serum ALT levels declined by week 28 by -3.0 U/L on empagliflozin vs -0.7 U/L on placebo and the changes were greatest in those with highest baseline ALT values).
- Bajaj HS, Brown RE, Bhullar L, Sohi N, Kalra S, Aronson R. SGLT2 inhibitors and incretin agents: Associations with alanine aminotransferase activity in type 2 diabetes. Diabetes Metab. 2018;44:493–9. [PubMed: 30149145](Among 3667 diabetic patients enrolled in a health care registry and started on antidiabetic therapies, serum ALT levels deceased with canagliflozin [-4.3 U/L], dapagliflozin [-3.5], sitagliptin [-1.8], liraglutide [-2.1] but not in controls [-0.3], and the decreases in ALT correlated with weight loss, but was greater with the SGLT2 inhibitors than the incretin agents).
- Choi DH, Jung CH, Mok JO, Kim CH, Kang SK, Kim BY. Effect of dapagliflozin on alanine aminotransferase improvement in type 2 diabetes mellitus with non-alcoholic fatty liver disease. Endocrinol Metab (Seoul). 2018;33:387–94. [PMC free article: PMC6145967] [PubMed: 30229578](Among 102 patients with type 2 diabetes and fatty liver disease started on metformin and either dapagliflozin or a dipeptidyl peptidase-4 inhibitor, ALT levels declined more with dapagliflozin [-21 vs -10 U/L] as did body weight [-2.9 vs -0.4 kg]).
- Itani T, Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes Sci Pract. 2018;4:477–82. [PMC free article: PMC6180715] [PubMed: 30338118](Among 35 patients with nonalcoholic fatty liver treated with canagliflozin, serum ALT levels decreased from a mean of 74 to 40 U/L at 6 months with concurrent decrease in body weight [73 to 70 kg] and HbA1c [7.5% to 6.4%]).
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [PubMed: 30415602](Among 17,160 patients with type 2 diabetes at risk for cardiovascular disease treated with dapagliflozin [10 mg] or placebo once daily for an average of 4.2 years, cardiovascular deaths and hospitalizations for heart failure were less with dapagliflozin, while discontinuations for adverse events were more common [8.1% vs 6.8%] as were genital infections [0.9% vs 0.1%] and diabetic ketoacidosis [0.3% vs 0.1%], but not amputations [1.4% vs 1.3%] or “hepatic events [both 1.0%]; no mention of ALT levels or hepatotoxicity).
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, et al. CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. [PubMed: 30990260](Among 4401 patients with diabetic nephropathy treated with canagliflozin [100 mg] of placebo daily for a median of 2.6 years, the risk of worsening of renal disease or death from renal or cardiovascular disease was reduced by canagliflozin, while adverse event rates were similar including low rates of “hepatic injury” [5.5 vs 6.5 per 1000 patient-years]; no mention of ALT elevations).
- Cardiovascular benefits of SGLT2 inhibitors and GLP-1 receptor agonists in Type 2 diabetes. Med Lett Drugs Ther. 2019;61(1566):26–28. [PubMed: 30845101](Concise update on the cardiovascular benefits of SGLT2 inhibitors and GLP-1 agonists mentions that long term therapy with canagliflozin and empagliflozin have been shown to reduce the risk of cardiovascular complications; no mention of hepatic adverse events).
- Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, Iijima M, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285–92. [PubMed: 30178600](Among 57 patients with type 2 diabetes and fatty liver treated with dapagliflozin [5 mg daily] or standard therapy for 24 weeks, serum ALT levels decreased with dapagliflozin [38 to 26.5 U/L] as did liver fat [CAP 315 to 290 dB/m], with little or no change in either with standard treatment).
- Halvorsen YD, Lock JP, Zhou W, Zhu F, Freeman MW. A 24-week, randomized, double-blind, active-controlled clinical trial comparing bexagliflozin with sitagliptin as an adjunct to metformin for the treatment of type 2 diabetes in adults. Diabetes Obes Metab. 2019;21:2248–2256. [PubMed: 31161692](Among 386 patients with type 2 diabetes with inadequate control on metformin given add on therapy for 24 weeks, there were similar improvements in HbA1c levels with bexagliflozin vs sitagliptin while total [71% vs 56%] and severe adverse event rates [3.7% vs 2.1%] were similar: no mention of ALT elevations or hepatotoxicity).
- Halvorsen YC, Walford GA, Massaro J, Aftring RP, Freeman MW. A 96-week, multinational, randomized, double-blind, parallel-group, clinical trial evaluating the safety and effectiveness of bexagliflozin as a monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. 2019;21:2496–2504. [PubMed: 31297965](Among 288 patients with type 2 diabetes treated with bexagliflozin or placebo for 96 weeks, HbA1c levels decreased with bexagliflozin but not with placebo, while adverse event rates were similar [including urinary tract infections and hypoglycemia] and a “hepatic” adverse event arose in one placebo- but no bexagliflozin-treated subject).
- Halvorsen YD, Walford G, Thurber T, Russell H, Massaro M, Freeman MW. A 12-week, randomized, double-blind, placebo-controlled, four-arm dose-finding phase 2 study evaluating bexagliflozin as monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. 2020;22:566–573. [PubMed: 31749238](Among 292 adults with type 2 diabetes treated with 3 different doses of bexagliflozin [5, 10 or 20 mg] or placebo daily for 12 weeks, there were dose related improvements in HbA1c and fasting blood glucose levels, and while total adverse event rates were similar with all 3 doses as with placebo, serious adverse events arose 5 bexagliflozin- but no placebo-treated subject: “assessment of the records of laboratory results…revealed no significant findings”).
- Shi FH, Li H, Yue J, Jiang YH, Gu ZC, Ma J, Lin HW. Clinical adverse events of high-dose vs low-dose sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of 51 randomized clinical trials. J Clin Endocrinol Metab. 2020; 105: dgaa586. [PubMed: 32841351](Metanalysis of 51 controlled trials of SGLT2 inhibitors for type 2 diabetes comparing 12,208 patients receiving high dose and 12,163 low dose therapy, overall and serious adverse event rates were similar and specific events mentioned infections, arthralgia, nausea, diarrhea, dizziness, creatinine elevations and hypoglycemia, but did not list or mention ALT elevations or hepatotoxicity).
- Drugs for type 2 diabetes. Med Lett Drugs Ther. 2022;64:177–184. [PubMed: 36384763](Concise review of the safety and efficacy of drugs approved for treatment of type 2 diabetes mentions adverse effects of genital mycotic infections, urinary tract infections, volume depletion, acute kidney injury diabetic ketoacidosis, and possible risk of lower limb amputations and bone fractures; but does not mention ALT elevations or hepatotoxicity).
- Halvorsen YD, Lock JP, Frias JP, Tinahones FJ, Dahl D, Conery AL, Freeman MW. A 96-week, double-blind, randomized controlled trial comparing bexagliflozin to glimepiride as an adjunct to metformin for the treatment of type 2 diabetes in adults. Diabetes Obes Metab. 2023;25:293–301. [PubMed: 36178197](Among 426 adults with type 2 diabetes with an inadequate response to metformin who received add on therapy with bexagliflozin [20 mg] or glimepiride [titrated] for 96 weeks, improvements in HbA1c levels were similar with both drugs, but episodes of hypoglycemia [17% vs 33%] were less with bexagliflozin, average body weight decreased [-3.75 kg vs +0.65 kg] and mean ALT levels improved [-4 U/L vs +1 U/L]; “hepatobiliary” adverse events occurred in 2.3% vs 2.8% of subjects).
- Koshino A, Oshima M, Arnott C, Fletcher RA, Bakris GL, Jardine M, Mahaffey KW, et al. Effects of canagliflozin on liver steatosis and fibrosis markers in patients with type 2 diabetes and chronic kidney disease: A post hoc analysis of the CREDENCE trial. Diabetes Obes Metab. 2023 Jan 19; Epub ahead of print. [PubMed: 36655422](Among 4387 patients with diabetic nephropathy treated with canagliflozin [100 mg] vs placebo daily for a median of 2.6 years, noninvasive measures of hepatic steatosis improved, and measures of hepatic fibrosis worsened less with canagliflozin, while ALT and AST levels did not differ between the two arms).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure.[Vasc Health Risk Manag. 2016]Review Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure.Reed JW. Vasc Health Risk Manag. 2016; 12:393-405. Epub 2016 Oct 27.
- Cardiovascular and renal outcomes with sodium glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus: A system review and network meta-analysis.[Front Pharmacol. 2022]Cardiovascular and renal outcomes with sodium glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus: A system review and network meta-analysis.Tian L, Ai S, Zheng H, Yang H, Zhou M, Tang J, Liu W, Zhao W, Wang Y. Front Pharmacol. 2022; 13:986186. Epub 2022 Nov 24.
- Review The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management.[Cardiovasc Diabetol. 2022]Review The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management.Xu B, Li S, Kang B, Zhou J. Cardiovasc Diabetol. 2022 May 25; 21(1):83. Epub 2022 May 25.
- Model-Based Evaluation of Proximal Sodium Reabsorption Through SGLT2 in Health and Diabetes and the Effect of Inhibition With Canagliflozin.[J Clin Pharmacol. 2018]Model-Based Evaluation of Proximal Sodium Reabsorption Through SGLT2 in Health and Diabetes and the Effect of Inhibition With Canagliflozin.Brady JA, Hallow KM. J Clin Pharmacol. 2018 Mar; 58(3):377-385. Epub 2017 Nov 16.
- Sodium-glucose cotransporter 2 inhibitors for the management of type 2 diabetes.[Expert Opin Pharmacother. 2021]Sodium-glucose cotransporter 2 inhibitors for the management of type 2 diabetes.Siamashvili M, Davis SN. Expert Opin Pharmacother. 2021 Nov; 22(16):2181-2198. Epub 2021 Aug 21.
- Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors - LiverToxSodium-Glucose Cotransporter-2 (SGLT2) Inhibitors - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...