NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Romidepsin is an intravenously administered histone deacetylase inhibitor and antineoplastic agent that is approved for use in refractory or relapsed cutaneous and peripheral T cell lymphomas. Romidepsin is associated with modest rate of minor serum enzyme elevations during therapy but has not been linked to cases of clinically apparent liver injury, although it has been reported to cause reactivation of hepatitis B.
Background
Romidepsin (roe" mi dep' sin) is an intravenously administered antineoplastic agent which acts by inhibition of histone deacetylases, thereby preventing removal of acetyl groups from histones. The accrual of acetyl groups on histones causes cell cycle arrest and apoptotic cell death. Malignant cells and particularly malignant T cells are particularly sensitive to the effects of inhibition of histone deacetylases. Romidepsin was initially isolated from a bacterium Chromobacterium violaceum and found to have antineoplastic activity. Elucidation of its molecular structure and analysis of its in vitro effects demonstrated that it was a histone deacetylase. Romidepsin has been evaluated as therapy of several malignancies and found to have greatest activity in T cell lymphomas. Romidepsin was approved for use in the United States in 2009 as monotherapy for refractory or relapsing cutaneous T cell lymphoma (CTCL) and indications were expanded in 2011 to include refractory or relapsing peripheral T cell lymphoma (PTCL). Romidepsin is available as a powder for reconstitution in 10 mg vials under the commercial name Istodax. The recommended dose is 14 mg/m2 given intravenously on days 1, 8 and 15 of a 28 day cycle. Side effects are common, but usually mild-to-moderate in severity, and include nausea, fatigue, fever, anemia, neutropenia, thrombocytopenia, constipation and rash. Side effects lead to early discontinuation in up to 15% of patients. Severe adverse events can include marked neutropenia, thrombocytopenia, serious infections, sepsis, tumor lysis syndrome and cardiac arrhythmias.
Hepatotoxicity
In clinical trials of romidepsin in patients with CTLC and PTLC, the rates of serum enzyme elevations during therapy ranged from 7% to 20%, but the abnormalities were usually transient and mild and did not require dose modifications. Serum ALT elevations above 5 times ULN occurred in 6% of patients. In the preregistration clinical trials of romidepsin, there were no reports of hepatitis, jaundice or clinically apparent liver injury among the treated subjects. Romidepsin has had limited clinical use, but there is no evidence that it is associated with significant liver injury.
Romidepsin also has immunomodulatory activities and has been reported to cause reactivation of latent DNA viruses including Epstein-Barr, varicella zoster and hepatitis B virus. Reactivation of hepatitis B occurred in a patient who was initially negative for HBsAg, but reactive for anti-HBc and anti-HBs. Nevertheless, the clinical features of hepatitis B reactivation were mild and responded to oral antiviral therapy. In patients with EBV associated lymphoma, romidepsin has been associated with severe reactivation of EBV infection and acute hepatitis that can be severe and even fatal.
Likelihood score: C (probable cause of clinically apparent liver injury, which can be due to reactivation of hepatitis B or EBV infection).
Mechanism of Injury
The reason why romidepsin might cause serum enzyme elevations is not known, but may be a direct toxicity to hepatocytes caused by inhibition of histone deacetylase or other enzyme activities. Romidepsin is metabolized in the liver by cytochrome P450 system, predominantly CYP 3A4 and is susceptible to drug-drug interactions. Reactivation of hepatitis B and EBV is likely due to the immunomodulatory actions of romidepsin.
Outcome and Management
Serum enzyme elevations during romidepsin therapy are usually mild and rarely dose limiting. Romidepsin should be held if ALT or AST values rise above 5 times the ULN and should be permanently discontinued if elevations exceed 20 times the ULN, or with the appearance of jaundice or symptoms of liver injury. There is no known cross sensitivity to hepatic injury among the different histone deacetylase inhibitors. Patients who are to receive romidepsin should be screened for evidence of hepatitis B and monitored carefully or given prophylaxis against HBV reactivation if they have evidence on ongoing or previous infection. Patients with active EBV infection should be treated with caution and with prophylaxis against reactivation.
Drug Class: Antineoplastic Agents, Histone Deacetylase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Romidepsin – Istodax®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
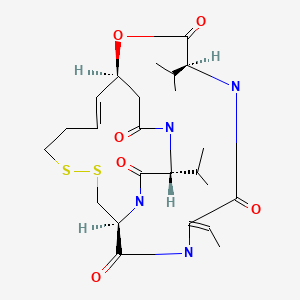
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Romidepsin | 128517-07-7 | C24-H36-N4-O6-S2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 September 2020
Abbreviation: PTCL, peripheral T-cell lymphoma.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of histone deacetylase inhibitors).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; does not discuss romidepsin).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Inhibitors of histone deacetylase. Pathway targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, p. 1230.(Textbook of pharmacology and therapeutics).
- Ritchie D, Piekarz RL, Blombery P, Karai LJ, Pittaluga S, Jaffe ES, Raffeld M, et al. Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report. Haematologica. 2009;94:1618–22. [PMC free article: PMC2770976] [PubMed: 19608677](Adult woman with PTCL refractory to conventional therapy received romidepsin and developed hepatitis after 11 cycles [bilirubin not given, ALT 226 U/L, HBsAg and HBeAg positive, IgM anti-HBc positive and HBV DNA 7.9 log10 copies/mL], biopsy showing moderate inflammation, and hepatitis resolving with entecavir therapy, but no follow up provided).
- Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, Zain J, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–7. [PMC free article: PMC2773225] [PubMed: 19826128](Among 71 patients with CTCL treated with romidepsin, the overall response rate was 34% and common side effects were nausea [52%], fatigue [41%]. vomiting [20%], anorexia [21%], leukopenia [31%], thrombocytopenia [39%], anemia [37%], infection [54%] and ALT or AST elevations [13%], but there were no episodes of clinically apparent liver injury).
- Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, Scarisbrick J, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–91. [PubMed: 20697094](Among 96 patients with CTCL treated with romidepsin, the overall response rate was 34% and adverse events were common, including gastrointestinal disturbance, fatigue, prolonged QTc, fever, tumor lysis syndrome and cardiac arrhythmias, but no one had ALT or AST elevations above 5 times ULN).
- Guan P, Fang H. Clinical development of histone deacetylase inhibitor romidepsin. Drug Discov Ther. 2010;4:388–91. [PubMed: 22491302](Review of the potential of histone deacetylase inhibitors as cancer chemotherapeutic agents states that romidepsin has a “tolerable toxicity profile”).
- Romidepsin (Istodax) for cutaneous T-cell lymphoma. Med Lett Drugs Ther. 2010;52(1339):42–3. [PubMed: 20508581](Concise review of the mechanism of action, clinical efficacy, safety, drug-drug interactions and costs of romidepsin therapy shortly after its approval in the US as monotherapy for refractory or relapsed CTCL).
- Cheson BD, Horwitz SM, Weisenburger DD. Peripheral T-cell lymphomas: diagnosis and treatment options. Proceedings from a live roundtable, August 17, 2011, Kauai, Hawaii. Clin Adv Hematol Oncol. 2011;9(11) Suppl 26:1–14. [PubMed: 22362328](Summary of the current status of the clinical features, natural history, diagnosis and management of PTCL).
- Jones SF, Infante JR, Spigel DR, Peacock NW, Thompson DS, Greco FA, McCulloch W, et al. Phase 1 results from a study of romidepsin in combination with gemcitabine in patients with advanced solid tumors. Cancer Invest. 2012;30:481–6. [PubMed: 22536933](Dose finding study of romidepsin and gemcitabine used in combination with paclitaxel for breast cancer, with cisplatin for NSCLC and with carboplatin for ovarian cancer; combinations that showed synergy in animal models).
- Kim M, Thompson LA, Wenger SD, O'Bryant CL. Romidepsin: a histone deacetylase inhibitor for refractory cutaneous T-cell lymphoma. Ann Pharmacother. 2012;46:1340–8. [PubMed: 22968522](Review of the pharmacology, clinically efficacy and safety of romidepsin mentions ALT or AST elevations occurred in 7-20% of patients which are above 5 times ULN in 6%).
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Borchmann P, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–6. [PubMed: 22271479](Among 130 patients with PTCL treated with romidepsin, the overall response rate was 25% and the most frequent serious adverse events were thrombocytopenia [24%], neutropenia [20%], and infections [19%]; no mention of ALT elevations or hepatotoxicity).
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. [PMC free article: PMC4016573] [PubMed: 24456586](Among 130 patients with PTCL treated with romidepsin, the overall response rate was 25% and adverse event rates decreased with long term therapy; no mention of ALT elevations or hepatotoxicity).
- Foss F, Coiffier B, Horwitz S, Pro B, Prince HM, Sokol L, Greenwood M, et al. Tolerability to romidepsin in patients with relapsed/refractory T-cell lymphoma. Biomark Res. 2014;2:16. [PMC free article: PMC4181623] [PubMed: 25279222](Combined analysis of the adverse events of romidepsin in trials of therapy in PTCL and CTCL focusing on gastrointestinal intolerance, cytopenias and serious infections; no mention of ALT elevations or hepatotoxicity).
- Dupuis J, Morschhauser F, Ghesquières H, Tilly H, Casasnovas O, Thieblemont C, Ribrag V, et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: a non-randomised, phase 1b/2 study. Lancet Haematol. 2015;2:e160–5. [PubMed: 26687958](Among 37 patients with PTCL treated with CHOP and romidepsin, 89% of patients had some degree of hematologic toxicity, serious adverse events occurred in 68%, and ALT or AST elevations arose in 49% which were above 5 times ULN in 8%).
- Zinzani PL, Pellegrini C, Cerciello G, Monaco F, Volpetti S, Peli A, Angelucci E, et al. Romidepsin in relapsed/refractory T-cell lymphomas: Italian experience and results of a named patient program. Leuk Lymphoma. 2016;57(10):2370–4. [PubMed: 26732313](Among 33 patients with refractory or relapsed T cell lymphomas treated with romidepsin, the overall response rate was 24% and adverse events led to early stopping in 23%; but no mention of ALT elevations or hepatotoxicity).
- Pellegrini C, Dodero A, Chiappella A, Monaco F, Degl'Innocenti D, Salvi F, Vitolo U, et al. Italian Lymphoma Foundation. (Fondazione Italiana Linfomi Onlus, FIL). A phase II study on the role of gemcitabine plus romidepsin (GEMRO regimen) in the treatment of relapsed/refractory peripheral T-cell lymphoma patients. J Hematol Oncol. 2016;9:38. [PMC free article: PMC4830040] [PubMed: 27071522](Among 20 patients with refractory or relapsed PTCL treated with romidepsin and gemcitabine, the overall response rate was 30% and adverse events were frequent including ALT or AST elevations in 20%, which were above 5 times ULN in 15%; no mention of clinically apparent liver injury).
- Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS. Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol. 2016;27:508–13. [PubMed: 26658891](Among 5 patients with extranodal NK T cell lymphoma treated with romidepsin, 3 developed evidence of EBV reactivation accompanied by elevations in AST [460-2600 U/L] and bilirubin [4-9 mg/dL], one dying of acute liver failure).
- Bringhen S, De Wit E, Dimopoulos MA. New agents in multiple myeloma: an examination of safety profiles. Clin Lymphoma Myeloma Leuk. 2017;17:391–407.e5. [PubMed: 28601492](Review of the safety profiles and adverse events of newer drugs for multiple myeloma including the histone deacetylase inhibitors – romidepsin, vorinostat and panobinostat, mentions that ALT elevations above 5 times ULN occur in 8% of patients treated with romidepsin but no mention of ALT elevations or hepatotoxicity with vorinostat or panobinostat).
- Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma. 2017;58:1306–19. [PubMed: 27813438](Extensive review of the mechanism of action, classification, clinical efficacy and safety of histone deacetylase inhibitors in T-cell lymphomas, mentions that romidepsin is a parenterally administered bicyclic peptide that is approved for use in refractory or relapsed cutaneous and peripheral T cell lymphoma, common adverse effects of which are nausea, fatigue, anemia, thrombocytopenia, neutropenia, infections, anorexia, hyperglycemia, constipation, pruritus, and ALT elevations).
- Duvic M, Bates SE, Piekarz R, Eisch R, Kim YH, Lerner A, Robak T, et al. Responses to romidepsin in patients with cutaneous T-cell lymphoma and prior treatment with systemic chemotherapy. Leuk Lymphoma. 2018;59:880–7. [PubMed: 28853310](Retrospective analysis of efficacy and safety of romidepsin in 96 previously treated patients with cutaneous T-cell lymphoma, found that severe adverse events were more frequent in those with previous treatment; no mention of ALT levels or hepatotoxicity).
- Shimony S, Horowitz N, Ribakovsky E, Rozovski U, Avigdor A, Zloto K, Berger T, et al. Romidepsin treatment for relapsed or refractory peripheral and cutaneous T-cell lymphoma: Real-life data from a national multicenter observational study. Hematol Oncol. 2019;37:569–77. [PubMed: 31674027](Among 53 patients with cutaneous or peripheral T cell lymphoma treated with romidepsin at 5 centers in Israel between 2013 and 2018, adverse events were frequent including anemia [86%], thrombocytopenia [69%], neutropenia [68%] and infections [36%], but there was no mention of ALT elevations or hepatotoxicity).
- Shah RR. Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Saf. 2019;42:235–45. [PubMed: 30649740](Review of the safety of histone deacetylase inhibitors approved for use in the US mentions that elevations in serum aminotransferase levels have been reported during therpy with romidepsin, panobinostat and belinostat but not with vorinostat and there have been no reports of clinically apparent hepatotoxicity, except for a single case of hepatic failure arising during a clinical trial of belinostat).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Romidepsin for the Treatment of Peripheral T-Cell Lymphoma.[Oncologist. 2015]Review Romidepsin for the Treatment of Peripheral T-Cell Lymphoma.Iyer SP, Foss FF. Oncologist. 2015 Sep; 20(9):1084-91. Epub 2015 Jun 22.
- Review Vorinostat.[LiverTox: Clinical and Researc...]Review Vorinostat.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Belinostat.[LiverTox: Clinical and Researc...]Review Belinostat.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin.[Ann Oncol. 2016]Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin.Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS. Ann Oncol. 2016 Mar; 27(3):508-13. Epub 2015 Dec 9.
- Review Romidepsin: a histone deacetylase inhibitor for refractory cutaneous T-cell lymphoma.[Ann Pharmacother. 2012]Review Romidepsin: a histone deacetylase inhibitor for refractory cutaneous T-cell lymphoma.Kim M, Thompson LA, Wenger SD, O'Bryant CL. Ann Pharmacother. 2012 Oct; 46(10):1340-8. Epub 2012 Sep 11.
- Romidepsin - LiverToxRomidepsin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...