NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Risperidone is an atypical antipsychotic that is used widely in the treatment of mania and schizophrenia. Risperidone therapy is associated with serum aminotransferase elevations and in rare instances has been linked to clinically apparent acute liver injury.
Background
Risperidone (ris per' i done) is a benzisoxazole derivative which appears to act as a dopamine type 2 (D2) and serotonin (5-HT2) receptor antagonist. Risperidone is indicated for treatment of schizophrenia and as monotherapy or combination therapy for acute manic or mixed episodes of bipolar I disorder in adults. Risperidone is also used for management of irritability with autistic disorder in children and adolescents. Risperidone was approved for use in the United States in 1993 and it is still widely used. Risperidone is available as tablets of 0.25, 0.5, 1, 2, 3 and 4 mg generically and under the brand name of Risperdal. Oral solutions for pediatric use are available as are orally disintegrating tablets and formulations for parenteral administration. The typical initial dose in adults is 1 mg once or twice daily, with increase in dose to as high as 8 mg daily based upon indications, efficacy and tolerance. Common side effects include somnolence, fatigue, restlessness, dizziness, dry mouth, increased saliva, constipation, increased appetite and weight gain. Rare, but potentially severe adverse events include cerebrovascular events, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, suicidal ideation and behavior, seizures, diabetes and agranulocytosis.
Hepatotoxicity
Liver test abnormalities may occur in up to 30% of patients on long term therapy with risperidone, usually arising within the first 8 weeks of treatment. The ALT elevations are usually mild, transient and may resolve even with continuation of medication. Instances of more marked ALT and alkaline phosphatase elevations, with or without symptoms and with or without jaundice, have also been reported. The onset of injury typically occurs within a few days of starting risperidone and resolves rapidly with stopping. Instances of acute liver injury with jaundice arising several months and even years after starting risperidone have also been reported. The pattern of serum enzyme elevations is typically cholestatic, but cases with hepatocellular and mixed patterns have also been described. Immunoallergic manifestations (rash, fever, eosinophilia) are rare; a case of autoimmune hepatitis apparently triggered by risperidone therapy has been published, but most cases do not have autoimmune features.
Likelihood score: B (likely but rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which risperidone causes serum ALT elevations is not known. Risperidone is extensively metabolized by the cytochrome P450 system (CYP 2D6) to its active metabolite. Some instances of liver injury may be due to nonalcoholic fatty liver disease caused by weight gain that occurs in at least one-quarter of treated patients and can be as much as 30 kg, generally during the first 1 to 2 years of therapy. In large series, however, the minor ALT elevations that occur on therapy have not correlated well with weight gain.
Outcome and Management
The serum aminotransferase elevations that occur on risperidone therapy are usually self-limited and often do not require dose modification or discontinuation of therapy. No instances of acute liver failure or vanishing bile duct syndrome have been attributed to risperidone. A single case of autoimmune hepatitis due to risperidone has been published. There may be some cross reactivity to liver injury between risperidone and quetiapine, but usually not with clozapine and olanzapine.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Risperidone – Generic, Risperdal®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
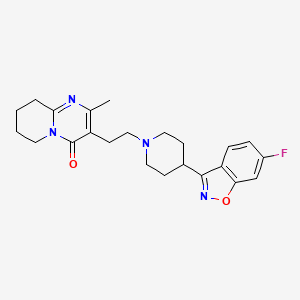
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Risperidone | 106266-06-2 | C23-H27-F-N4-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 06 June 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Larry D. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 507-26.(Review of hepatotoxicity of psychiatric agents mentions that at least 10 cases of liver injury have been ascribed to risperidone, some with steatosis and weight gain and others with acute clinical jaundice).
- Risperidone for chronic schizophrenia. Med Lett Drugs Ther. 1994;36:33–4. [PubMed: 7511777](Brief review of efficacy and safety of risperidone shortly after its approval in the US; common side effects are asthenia, sedation and difficulty concentrating, orthostatic hypotension and weight gain; no mention of liver injury).
- Fuller MA, Simon MR, Freedman L. Risperidone-associated hepatotoxicity. J Clin Psychopharmacol. 1996;16:84–5. [PubMed: 8834428](Two cases; 64 year old man developed jaundice 6 weeks after starting risperidone [bilirubin 16.1 mg/dL, ALT 297 U/L, Alk P 119 U/L], resolving upon stopping; 68 year old woman developed lethargy 1 year after starting risperidone [bilirubin 0.7 mg/dL, ALT 341 U/L, Alk P 174 U/L], resolving rapidly upon stopping).
- Kumra S, Herion D, Jacobsen LK, Briguglia C, Grothe D. Case study: risperidone-induced hepatotoxicity in pediatric patients. J Am Acad Child Adolesc Psychiatry. 1997;36:701–5. [PubMed: 9136506](Retrospective review of 13 children on risperidone; 2 developed elevated ALT levels [108 and 144 U/L] with weight gain [16 and 5 kg] on long term risperidone [7 and 17 months], resolution on stopping risperidone even without much weight loss).
- Geller WK, Zuiderwijk PB. Risperidone-induced hepatotoxicity? J Am Acad Child Adolesc Psychiatry. 1998;37:246–7. [PubMed: 9519624](Letter disagreeing with comments of Kumra [1997], arguing against routine ALT monitoring for patients on risperidone).
- Landau J, Martin A. Is liver function monitoring warranted during risperidone treatment? J Am Acad Child Adolesc Psychiatry. 1998;37(10):1007–8. [PubMed: 9785708](Overweight 13 year old girl found to have ALT elevations [164 U/L] 3 days after starting risperidone and steatohepatitis on biopsy; no values before starting therapy).
- Benazzi F. Risperidone-induced hepatotoxicity. Pharmacopsychiatry. 1998;31:241. [PubMed: 9930641](25 year old woman treated with increasing doses of risperidone [to 6 mg/day] had 20 kg weight gain and fatigue; ALT rose from 13 [pre] to 277 U/L [month 4] without jaundice and fell to normal 1 month after stopping drug).
- Phillips EJ, Liu BA, Knowles SR. Rapid onset of risperidone-induced hepatotoxicity. Ann Pharmacother. 1998;32:843–4. [PubMed: 9681106](81 year old man developed jaundice within 2 days of starting risperidone [bilirubin 3.6 mg/dL, ALT 101 U/L, Alk P 244 U/L], resolving 2 weeks after stopping).
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. [PubMed: 10553730](Systematic review of 81 articles on weight change with antipsychotics, using change after 10 weeks to compare agents: clozapine +5.7, olanzapine +4.2, chlorpromazine +4.2, quetiapine +2.5, risperidone +1.7, loxapine +0.6, haloperidol +0.5, ziprasidone +0.3, molindone -0.1, and pimozide -2.7 kg).
- Whitworth AB, Liensberger D, Fleischhacker WW. Transient increase of liver enzymes induced by risperidone: two case reports. J Clin Psychopharmacol. 1999;19:475–6. [PubMed: 10505592](Two cases; 22 year old man had ALT elevation [106 U/L] 16 days after starting risperidone, which improved with lowering dose from 6 to 5 mg/day; 19 year old man had ALT elevation [43 U/L] which fell to normal despite continuing risperidone therapy).
- Szigethy E, Wiznitzer M, Branicky LA, Maxwell K, Findling RL. Risperidone-induced hepatotoxicity in children and adolescents? A chart review study. J Child Adolesc Psychopharmacol. 1999;9:93–8. [PubMed: 10461819](Retrospective study of 38 children and adolescents on risperidone for ~15 months; only 1 had ALT elevation [46 U/L], degree of surveillance unclear; abstract only).
- Conley RR, Conley RR. Risperidone side effects. J Clin Psychiatry. 2000;61 Suppl 8:20–3. [PubMed: 10811239](Review of side effects of risperidone, but no mention of hepatotoxicity or ALT elevations).
- Krebs S, Dormann H, Muth-Selbach U, Hahn EG, Brune K, Schneider HT. Risperidone-induced cholestatic hepatitis. Eur J Gastroenterol Hepatol. 2001;13:67–9. [PubMed: 11204814](37 year old man developed rises in ALT [140 U/L] and GGT [260 U/L] within days of starting risperidone, that continued on haloperidol [ALT 100 U/L] and olanzapine [ALT 160 U/L]; peak bilirubin 1.6 mg/dL; biopsy showed “cholestatic hepatitis”; tests fell to normal ~1 month after discontinuation).
- Cordeiro Q Jr, Elkis H. Pancreatitis and cholestatic hepatitis induced by risperidone. J Clin Psychopharmacol. 2001;21:529–30. [PubMed: 11593080](32 year old man developed abdominal pain and jaundice 1 week after starting risperidone [bilirubin 2.8 mg/dL, ALT 366 U/L, Alk P 367 U/L, amylase 1617 U/L], resolving rapidly with stopping).
- Dumortier G, Cabaret W, Stamatiadis L, Saba G, Benadhira R, Rocamora JF, Aubriot-Delmas B, et al. Encephale. 2002;28:542–51. [Hepatic tolerance of atypical antipsychotic drugs] [PubMed: 12506267](Review of literature on liver injury due to atypical antipsychotics).
- Mouradian-Stamatiadis L, Dumortier G, Januel D, Delmas BA, Cabaret W. Liver function tests during treatment with antipsychotic drugs: a case series of 23 patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1409–11. [PubMed: 12502031](23 hospitalized patients on atypical antipsychotics, 6 had ALT or AST elevations by day 14, ALT 48-158 U/L, 2 on risperidone, 2 on olanzapine and one on amisulpride; 1 on risperidone required discontinuation).
- Haberfellner EM, Honsig T. Nonalcoholic steatohepatitis: a possible side effect of atypical antipsychotics. J Clin Psychiatry. 2003;64:851. [PubMed: 12934993](Three patients with weight gain [24-26 kg] had ALT elevations [47-91 U/L] on olanzapine or risperidone for ~4 years; ultrasound suggested fatty liver).
- Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28:99–112. [PMC free article: PMC161731] [PubMed: 12670127](Review of the interactions of the atypical antipsychotics with the P450 system; clozapine is metabolized by CYP1A2 and 3A4 and possibly 2C9 and 2D6; risperidone by CYP2D6 and possibly 3A4; olanzapine by CYP1A2 and possibly 2D6; quetiapine and ziprasidone by CYP3A4).
- Bender S, Grohmann R, Engel RR, Degner D, Dittmann-Balcar A, Ruther E. Severe adverse drug reactions in psychiatric inpatients treated with neuroleptics. Pharmacopsychiatry. 2004;37 Suppl 1:S46–53. [PubMed: 15052514](Severe adverse drug reactions among 35,293 inpatients; adverse events more common with atypicals [0.5-0.9%] than typical antipsychotic agents [0.02-0.2%], increased liver enzymes was the most common adverse reaction to olanzapine, 4th in frequency to clozapine, 6th to haloperidol, 7th to risperidone; no mention of hepatitis or acute liver failure).
- Holtmann M, Kopf D, Mayer M, Bechtinger E, Schmidt MH. Risperidone-associated steatohepatitis and excessive weight-gain. Pharmacopsychiatry. 2003;36:206–7. [PubMed: 14571356](17 year old woman had significant weight gain [35 kg] and ALT elevations [108 U/L] on clozapine that worsened when switched to risperidone; ultrasound showed hypo-echogenicity suggestive of fatty liver; ALT levels decreased after stopping risperidone and a weight loss of 5 kg).
- Choice of an antipsychotic. Med Lett Drugs Ther. 2003;45:102–4. [PubMed: 14679353](Risperidone is a second generation antipsychotic with the highest rate of galactorrhea, gynecomastia and sexual dysfunction; no mention of liver injury).
- Aman MG, Arnold LE, McDougle CJ, Vitiello B, Scahill L, Davies M, McCracken JT, et al. Acute and long-term safety and tolerability of risperidone in children with autism. J Child Adolesc Psychopharmacol. 2005;15:869–84. [PubMed: 16379507](8 week trial of risperidone vs placebo in 101 children and adolescents; ALT levels rose to >2 times ULN in 2 risperidone- vs no placebo-recipients).
- Llinares Tello F, Hernández Prats C, Bosacoma Ros N, Pérez Martínez E, Climent Grana E, Navarro Polo JN, Ordovás Baines JP. Acute cholestatic hepatitis probably associated with risperidone. Int J Psychiatry Med. 2005;35:199–205. [PubMed: 16240976](64 year old man developed rising serum enzymes between days 2 and 5 of risperidone treatment [bilirubin 0.5 mg/dL, ALT 186 U/L, Alk P 1326 U/L], values falling to near normal 2 weeks after stopping).
- Radzik J, Grotthus B, Leszek J. Psychiatr Pol. 2005;39:309–13. [Disorder of liver functions in a schizophrenic patient after long-term risperidone treatment—case report] [PubMed: 15881625](51 year old man developed jaundice after 4 years of risperidone therapy [bilirubin 2.5 mg/dL, AST 49 U/L, normal Alk P and GGT], possibly representing Gilbert syndrome and minor AST elevations from risperidone; unclear whether abnormalities resolved on stopping).
- Pae CU, Lim HK, Kim TS, Kim JJ, Lee CU, Lee SJ, Lee C, et al. Naturalistic observation on the hepatic enzyme changes in patients treated with either risperidone or olanzapine alone. Int Clin Psychopharmacol. 2005;20:173–6. [PubMed: 15812269](Retrospective analysis found ALT elevations more common among 145 patients on olanzapine [25%] than 298 on risperidone [13%] and particularly >3 times ULN [7.6% vs 2.8%]; no instances of jaundice or hepatitis).
- Esposito D, Brocvielle H, Becquemont L, Hardy P, Chouinard G, Corruble E. Risperidone-induced immunoallergic hepatitis. Am J Psychiatry. 2005;162:1984. [PubMed: 16199856](28 year old man developed enzyme elevations 7 weeks and symptoms 8 weeks after starting risperidone [bilirubin normal, ALT 522 U/L], resolving within 3 weeks of stopping; smooth muscle antibody positive).
- Legaz Huidobro ML, González Carro P, Pérez Roldán F, Soto Fernández S, de Pedro Esteban A, Cuesta Domínguez R. Gastroenterol Hepatol. 2005;28:135–6. [Autoimmune hepatitis after risperidone-induced cholestatic hepatitis] Spanish. [PubMed: 15771859](36 year old woman developed jaundice 10 days after starting risperidone [bilirubin rising from 15 to 29 mg/dL, ALT 818 U/L, Alk P 627 U/L, ANA of 1:320]; ALT continued to rise for 3 weeks [peak 1185 U/L] and prednisone was started with rapid response, but relapse [ALT 843 U/L, ANA 1:1280] when corticosteroids stopped, biopsy showing features of autoimmune hepatitis which was again controlled by prednisone and later with azathioprine alone).
- Perlis RH, Baker RW, Zarate CA Jr, Brown EB, Schuh LM, Jamal HH, Tohen M. Olanzapine versus risperidone in the treatment of manic or mixed States in bipolar I disorder: a randomized, double-blind trial. J Clin Psychiatry. 2006;67:1747–53. [PubMed: 17196055](Trial of 3 weeks of olanzapine [n=165] vs risperidone [n=164] for bipolar illness; greater weight gain [16% vs 4%] and ALT elevations [mean increase 15.7 U/L vs 1 U/L] with olanzapine).
- Rettenbacher MA, Baumgartner S, Eder-Ischia U, Edlinger M, Graziadei I, Hofer A, Huber R, et al. Association between antipsychotic-induced elevation of liver enzymes and weight gain: a prospective study. J Clin Psychopharmacol. 2006;26:500–3. [PubMed: 16974192](Prospective study of 67 patients started on atypical antipsychotics [10 on risperidone]; ALT elevations were more frequent in 14 patients who gained >7% of body weight than in 53 who did not [50% vs 19%] and mean changes in ALT, AST and GGT were greater; all were transient, asymptomatic and not associated with bilirubin elevations).
- Wright TM, Vandenberg AM. Risperidone- and quetiapine-induced cholestasis. Ann Pharmacother. 2007;41:1518–23. [PubMed: 17666578](30 year old man developed jaundice after 8 years of therapy with risperidone and lithium [bilirubin 4.7 mg/dL, ALT 99 U/L, Alk P 267 U/L], resolving with change of risperidone to ziprasidone, but recurring 3 weeks after starting quetiapine, after tolerating olanzapine).
- Atasoy N, Erdogan A, Yalug I, Ozturk U, Konuk N, Atik L, Ustundag Y. A review of liver function tests during treatment with atypical antipsychotic drugs: a chart review study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1255–60. [PubMed: 17600607](Retrospective analysis on 194 patients on atypical antipsychotics; ALT >3 times ULN occurred in 27%, often in first month; among 29 receiving risperidone, 28% had ALT elevations, but elevations were modest and none >3 times ULN).
- Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, Ambler D, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420–31. [PubMed: 18794207](Prospective trial of molindone [1st generation antipsychotic agent] vs olanzapine or risperidone [2nd generation agents] for schizophrenia in 199 youths found similar rates of efficacy [34-50%], but more weight gain [mean 6.1 kg] and ALT elevations with olanzapine).
- Erdogan A, Atasoy N, Akkurt H, Ozturk D, Karaahmet E, Yalug I, Yalug K, et al. Risperidone and liver function tests in children and adolescents: a short-term prospective study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:849–57. [PubMed: 18258348](Among 120 children and adolescents prospectively followed for one month on risperidone therapy, 52.5% had mild ALT elevations, but only 1 subject had ALT >3 times ULN [ALT 225 U/L, Alk P 641 U/L, bilirubin 0.4 mg/dL], resolving with discontinuation; no correlation with weight gain, which occurred in 57%).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008; several antidepressants [duloxetine, sertaline, fluoxetine, amitryptilline], but none of the atypical antipsychotic agents were implicated).
- Torrent C, Amann B, Sanchez-Moreno J, Colom F, Feinares M, Comes M, Rosa AR, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18. [PubMed: 18498432](Review of frequency of weight gain in patients treated for bipolar disorders, most weight gain occurred with clozapine and olanzapine, but some weight gain also with quetiapine, risperidone, lithium, valproate and gabapentin; not with aripiprazole, ziprasidone, carbamazepine or lamotrigine).
- Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–10. [PubMed: 19321312](Analysis of weight gain in 21 placebo controlled trials [~3300 patients]; average monthly weight gain in pounds was +0.1 with placebo, +0.8 olanzapine, 0.6 risperidone, -0.3 ziprasidone; a 5% increase in weight occurred after one year in 13% of placebo, 39% haloperidol, 20% ziprasidone, 45% risperidone and 60% olanzapine treated subjects).
- Paulzen M, Orfanos S, Gründer G. Remission of drug-induced hepatitis after switching from risperidone to paliperidone. Am J Psychiatry. 2010;167:351–2. [PubMed: 20194492](43 year old woman with schizophrenia developed jaundice on risperidone and clozapine therapy [bilirubin not given, ALT 75 U/L, Alk P 6 times ULN], improving on switching from risperidone to paliperidone and lowering clozapine dosage).
- Lin CH, Kuo CC, Chou LS, Chen YH, Chen CC, Huang KH, Lane HY. A randomized, double-blind comparison of risperidone versus low-dose risperidone plus low-dose haloperidol in treating schizophrenia. J Clin Psychopharmacol. 2010;30:518–25. [PubMed: 20814315](Controlled trial of 6 weeks of risperidone alone vs lower dose risperidone and haloperidol in 88 patients with schizophrenia; no change in mean ALT or AST levels in either group and no mention of hepatotoxicity).
- Erdogan A, Karaman MG, Ozdemir E, Yurteri N, Tufan AE, Kurcer MA. Six months of treatment with risperidone may be associated with nonsignificant abnormalities of liver function tests in children and adolescents: a longitudinal, observational study from Turkey. J Child Adolesc Psychopharmacol. 2010;20:407–13. [PubMed: 20973711](Among 102 children or adolescents [ages 2-18 years] treated with risperidone for more than 6 months, serum enzyme elevations occurred in 38%, but were usually transient and mild and isolated Alk P elevations only; only one child had ALT rise above twice normal [ALT 225 U/L, GGT 27 U/L, bilirubin 0.5 mg/dL], which resolved within one month of stopping; weight gain averaged 5.5 kg at 6 months).
- Karaman MG, Erdoğan A, Tufan E, Yurteri N, Ozdemir E, Ankarali H. Liver function tests in children and adolescents receiving risperidone treatment for a year: a longitudinal, observational study from Turkey. Int J Psychiatry Clin Pract. 2011;15:204–8. [PubMed: 22121930](Among 100 children or adolescents treated with risperidone for more than 6 months, liver tests were abnormal in 21%, but none had symptoms and only one child had an ALT level above 3 times ULN, which resolved rapidly upon stopping).
- Copur M, Erdogan A. Risperidone rechallenge for marked liver function test abnormalities in an autistic child. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:237–9. [PubMed: 21913889](5 year old boy with autism was found to have elevations in serum enzymes during risperidone therapy, which resolved on stopping, but did not recur when risperidone was restarted).
- Greil W, Häberle A, Schuhmann T, Grohmann R, Baumann P. Age and adverse drug reactions from psychopharmacological treatment: data from the AMSP drug surveillance programme in Switzerland. Swiss Med Wkly. 2013;143:w13772. [PubMed: 23821346](Among 39,728 patients treated with psychiatric medications in Swiss hospitals between 2001 and 2010 who were monitored in a drug surveillance program, rates of severe adverse events were similar in those above and below the age of 60 [1.6% vs 1.8%], although weight gain and ALT elevations were less frequent in the elderly [details not provided]).
- López-Torres E, Süveges A. Peñas-LLedó EM, Doña A, Dorado P, LLerena A, Berecz R. Liver enzyme abnormalities during antipsychotic treatment: a case report of risperidone-associated hepatotoxicity. Drug Metabol Drug Interact. 2014;29:123–6. [PubMed: 24598833](19 year old man with schizophrenia developed fatigue and weight loss 3 weeks after starting risperidone [bilirubin normal, ALT 778, Alk P normal], resolving within 2 months of stopping).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to risperidone or other atypical antipsychotic medications).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, five were attributed to atypical antipsychotics [3 quetiapine, 2 olanzapine], but none to risperidone).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that olanzapine can cause aminotransferase elevations, and that olanzapine and ziprasidone can cause DRESS syndrome, but does not mention ALT elevations or hepatotoxicity for any of agents discussed, including aripiprazole, brexpiprazole, cariprazine, clozapine, quetiapine, risperidone, asenapine, iloperidone, paliperidone and lurasidone).
- Morlán-Coarasa MJ, Arias-Loste MT, Ortiz-García de la Foz V, Martínez-García O, Alonso-Martín C, Crespo J, Romero-Gómez M, et al. Incidence of non-alcoholic fatty liver disease and metabolic dysfunction in first episode schizophrenia and related psychotic disorders: a 3-year prospective randomized interventional study. Psychopharmacology (Berl). 2016;233:3947–52. [PubMed: 27620899](Among 191 schizophrenic patients treated with an atypical antipsychotic agent for at least 3 years, surrogate markers for steatosis arose in 48 [25%], most of whom had a 7% increase in body weight [n=44: 92%], increase in triglycerides [54%], total cholesterol [52%], and waist circumference [68%]; changes in regard to fatty liver did not vary by specific antipsychotic agent [12 received risperidone]).
- Saglam O, Bahsi R, Akkoca Y, Filik L. Risperidone-induced hepatotoxicity in a patient addicted to synthetic cannabinoid. Eur J Gastroenterol Hepatol. 2016;28:360–1. [PubMed: 26825144](31 year old man developed fatigue 3 months after starting risperidone to treat cannabinoid addiction [bilirubin 2.3 mg/dL, ALT 890 U/L, Alk P 736 U/L], resolving within 2 weeks of stopping).
- Baeza I, de la Serna E, Calvo-Escalona R, Merchán-Naranjo J, Rodríguez-Latorre P, Martínez-Cantarero MC, Andrés P, et al. One-year prospective study of liver function tests in children and adolescents on second-generation antipsychotics: is there a link with metabolic syndrome? J Child Adolesc Psychopharmacol. 2018;28:463–73. [PubMed: 29975563](Among 216 children and adolescents starting atypical antipsychotics, mean weight gain at 6 months was 6.5 kg and mean ALT levels increased by 8.6 U/L, while among 69 taking risperidone mean weight gain was 8.3 kg and ALT increase 2.7 U/L; increases in ALT associated most closely with development of the metabolic syndrome, mean ALT increasing by 27.8 U/L at 6 months).
- Khorassani F, Sousonis F, Lopez LV. Risperidone- and paliperidone-induced hepatotoxicity: case report and review of literature. Am J Health Syst Pharm. 2020;77:1578–1584. [PubMed: 32699878](23 year old man with schizophrenia developed liver test abnormalities 13 days after starting risperidone [2 rising to 6 mg daily] with serum ALT rising from 25 to 469 U/L, falling when risperidone was stopped but rising again upon starting intramuscular paliperidone [234 mg], then gradually decreasing with a normal ALT 1 month later).
- Druschky K, Toto S, Bleich S, Baumgärtner J, Engel RR, Grohmann R, Maier HB, et al. Severe drug-induced liver injury in patients under treatment with antipsychotic drugs: data from the AMSP study. World J Biol Psychiatry. 2021;22:373–386. [PubMed: 32892689](Among 246 cases of severe liver injury due to antipsychotic medications identified in a prospective registry of German psychiatric hospitals between 1993 and 2016, 46 arose in 38,349 patients [0.12%] who received clozapine [34 as a single antipsychotic agent]; other commonly implicated agents being olanzapine [n=90 of 54,822: 0.16%], quetiapine [34 of 66,209: 0.05%] and risperidone [27 of 51,683: 0.05%]; two fatal cases occurred in olanzapine-treated patients).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine, but none for risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine).
- Gunther M, Dopheide JA. Antipsychotic safety in liver disease: a narrative review and practical guide for the clinician. J Acad Consult Liaison Psychiatry. 2023;64:73–82. [PubMed: 36180017](Review of the literature on hepatotoxicity of antipsychotic medications and guidance on their use in patients with liver disease characterizes chlorpromazine, clozapine, and olanzapine as having the greatest risk for causing liver injury, quetiapine and risperidone as having moderate risk, haloperidol as having low risk and paliperidone, aripiprazole, lurasidone, and loxapine as having low risk).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review [Antipsychotics in bipolar disorders].[Encephale. 2004]Review [Antipsychotics in bipolar disorders].Vacheron-Trystram MN, Braitman A, Cheref S, Auffray L. Encephale. 2004 Sep-Oct; 30(5):417-24.
- Review Olanzapine.[LiverTox: Clinical and Researc...]Review Olanzapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Clozapine.[LiverTox: Clinical and Researc...]Review Clozapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Mania, glutamate/glutamine and risperidone in pediatric bipolar disorder: a proton magnetic resonance spectroscopy study of the anterior cingulate cortex.[J Affect Disord. 2007]Mania, glutamate/glutamine and risperidone in pediatric bipolar disorder: a proton magnetic resonance spectroscopy study of the anterior cingulate cortex.Moore CM, Biederman J, Wozniak J, Mick E, Aleardi M, Wardrop M, Dougherty M, Harpold T, Hammerness P, Randall E, et al. J Affect Disord. 2007 Apr; 99(1-3):19-25. Epub 2006 Sep 26.
- Risperidone in the treatment of bipolar mania.[Neuropsychiatr Dis Treat. 2006]Risperidone in the treatment of bipolar mania.Sajatovic M, Subramoniam M, Fuller MA. Neuropsychiatr Dis Treat. 2006 Jun; 2(2):127-38.
- Risperidone - LiverToxRisperidone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...