NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Resmetirom is a thyroid hormone receptor beta (THR-β) agonist used in conjunction with diet and exercise in the therapy of nonalcoholic steatohepatitis (NASH) with moderate-to-severe fibrosis. Resmetirom therapy is associated with mild and transient serum aminotransferase elevations during the first month of therapy and with rare instances of acute liver injury which can be severe, but which reverses on drug discontinuation.
Background
Resmetirom (res” me tir’ om) is a thyroid hormone receptor beta (THR-β) agonist used in the therapy of nonalcoholic steatohepatitis (NASH) with moderate-to-severe fibrosis. Resmetirom is a triiodothyronine (T3) mimetic agent that has preferential activity against the thyroid hormone receptor β (the predominant form found in the liver) and minimal activity against the receptor α (the major form found in heart and bone). In the liver, engagement of THR-β results in a decrease in lipogenesis, increase in fatty acid β oxidation, and decrease in hepatic fat accompanied by improvements in serum lipid profiles. In clinical trials in patients with NASH, resmetirom resulted in a decrease in hepatic fat as well as inflammation and cell injury. When given for 52 weeks, resmetirom was associated with a modest improvement in hepatic fibrosis. Resmetirom was approved in the United States in 2024 in conjunction with diet and exercise as therapy for adults with noncirrhotic NASH and moderate to advanced fibrosis. Similar trials in children and adolescents and in adults with cirrhosis are ongoing. Resmetirom is available in tablets of 60, 80, and 100 mg under the brand name Rezdiffra. The recommended daily dose is 80 mg for adults weighing less than 100 kg and 100 mg for those weighing 100 kg or more. Side effects of resmetirom include diarrhea, gastrointestinal discomfort, nausea, constipation, headache, dizziness, pruritus, and rash. Changes in thyroid function and decreases in sex hormones as well as follicle stimulating and luteinizing hormones can occur with therapy but do not appear to be clinically significant. Serious adverse events are rare but can include cholecystitis, pancreatitis, and hepatotoxicity.
Hepatotoxicity
Mild, transient serum aminotransferase elevations develop in a high proportion of patients receiving resmetirom, generally within the first 4 weeks of therapy. These elevations are typically mild, self-limited, and not associated with symptoms or jaundice. Furthermore, these early changes were usually followed by a decrease in serum enzymes which were often within normal range 3 to 6 months later. These improvements in liver related enzymes correlated to some extent with the decrease in hepatic fat and histologic evidence of steatohepatitis. After 52 weeks of treatment, liver biopsies demonstrated resolution of NASH in 26% to 30% of patients. Whether these changes are sustained or increase with further therapy is not known. Therapy does not result in weight loss, and the improvements in hepatic histology and fibrosis may be lost once therapy is discontinued.
Analysis of liver tests from more than 1300 adults with NASH treated with resmetirom in doses of 80 or 100 mg daily for up to one year identified 2 patients with liver injury that was considered at least possibly due to resmetirom. The latency to initial onset was 2 and 3 months [ALT 236 U/L and 578 U/L, Alk P unknown and 64 U/L, bilirubin 0.6 and 1.1 mg/dL]. Both patients recovered completely within 1 to 2 months of stopping treatment. One patient was restarted on treatment and redeveloped liver injury within 28 days (ALT 3226 U/L, Alk P 140 U/L, bilirubin 10.9 mg/dL) that was more severe than the initial episode, but that resolved spontaneously within 2 months of stopping. In both cases, other diagnoses remained possible.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of liver injury from resmetirom is not known, but in the two reported cases, the injury appeared to be idiosyncratic and possibly immunologically mediated. Resmetirom is metabolized in part by the liver, predominantly by CYP 2C8 and concomitant use of strong CYP 2C8 inhibitors should be avoided.
Outcome and Management
The product label for resmetirom recommends monitoring of liver tests during therapy, but does not provide guidance regarding timing or duration of monitoring. In view of the preliminary data on hepatic tests during resmetirom therapy, an appropriate approach would be testing at baseline and at 1, 2, 3 and 6 months, and every 6 months thereafter. Such testing also provides information on both the response of the liver disease as well as hepatic safety. Rise in serum aminotransferase levels by more than 3 times the ULN or three times baseline levels should lead to increased monitoring or temporary discontinuation until levels return to normal or baseline. Elevations of serum aminotransferase levels accompanied by symptoms or jaundice should lead to prompt discontinuation. There is no evidence of cross sensitivity to hepatotoxicity of resmetirom to other therapies used for NASH or liver disease.
Drug Class: Antilipemic Agents, Drugs for Liver Disease
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Resmetirom – Rezdiffra®
DRUG CLASS
Drugs for Liver Disease
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Resmetirom | 920509-32-6 | C17-H12-Cl2-N6-O4 |

|
| Triiodothyronine | 6893-02-3 | C15-H12-I3-NO4 |
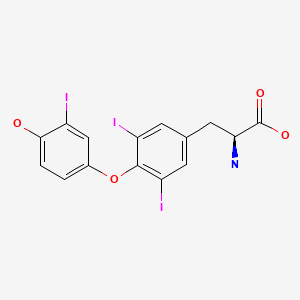
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 April 2024
Abbreviations: NASH, nonalcoholic steatohepatitis; THR, thyroid hormone receptor.
- FDA. Resmetirom, Integrated Review. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2024/217785Orig1s000IntegratedR.pdf (FDA Summary of the data on resmetirom safety and efficacy submitted in support of the application for approval as therapy of nonalcoholic steatohepatitis (NASH) mentions that one probable and one possible case of drug induced liver disease arose among the 1333 patients included in two prospective trials used in the safety assessment; the probable case was in a 60 year old woman who developed liver test abnormalities 57 days after starting resmetirom [ALT 236 U/L, AlkP not done, bilirubin 0.4 mg/dL], which were slow to improve after stopping but rose from normal to high levels within a month of restarting resmetirom [ALT 3226 U/L, Alk P 140 U/L, bilirubin 10.9 mg/dL] which again fell to normal within 2 months of stopping without corticosteroid therapy). - Kelly MJ, Pietranico-Cole S, Larigan JD, Haynes NE, Reynolds CH, Scott N, Vermeulen J, et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014; 57: 3912-23. [PubMed: 24712661](Description of development of thyroid hormone [TH] mimetic chemical structures that had selective affinity for the β receptors [THR-β] which are found predominantly on hepatocytes, the optimal molecule [MGL-3196: resmetirom] showed 84% of the relative activity of T3 against the β receptor but only 49% of that against the α receptor, and in animal models it had cholesterol and triglyceride lowering activity with no cardiac toxicity or effect on the central thyroid axis).
- Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019; 394: 2012-2024. [PubMed: 31727409](Among 125 adults with biopsy confirmed non-cirrhotic NASH treated with resmetirom [60-80 mg daily] or placebo once daily for 36 weeks, MRI estimated liver fat fraction decreased more with resmetirom as did liver biopsy fat, inflammation and ballooning, and serum ALT and AST levels, while adverse events were similar in both groups except for symptoms of diarrhea and nausea; no mention of hepatotoxicity or liver injury with jaundice).
- Harrison SA, Bashir M, Moussa SE, McCarty K, Pablo Frias J, Taub R, Alkhouri N. Effects of resmetirom on noninvasive endpoints in a 36-week phase 2 active treatment extension study in patients with NASH. Hepatol Commun. 2021; 5: 573-588. [PMC free article: PMC8034581] [PubMed: 33860116](Among 125 patients with NASH participating in a 36-week randomized placebo-controlled trial, 31 were enrolled in an open-label extension study for another 36 weeks which demonstrated a further decline in the liver fat fraction as well as improvements in serum aminotransferase levels and lipids, with no weight loss and no treatment related severe adverse events).
- Karim G, Bansal MB. Resmetirom: an orally administered, small molecule, liver-directed, β-selective THR agonist for the treatment of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. touchREV Endocrinol. 2023; 19: 60-70. [PMC free article: PMC10258622] [PubMed: 37313239](Review of the mechanism of action and clinical effects of resmetirom, a relatively selective thyroid hormone receptor beta agonist, which decreases hepatic fat and lipotoxicity, and holds promise as therapy of NASH).
- Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, et al.; MAESTRO-NASH Investigators. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024; 390: 497-509. [PubMed: 38324483](Among 966 patients with NASH and significant fibrosis treated with resmetirom [80 or 100 mg] or placebo once daily for 52 weeks, both doses were associated with a greater degree of improvement in liver histology [fibrosis, steatosis, ballooning and inflammation] than placebo, and while diarrhea, nausea, and vomiting were more frequent with resmetirom, they were transient and both total and serious adverse event rates were similar in the three groups and “there was no incidence of drug-induced liver injury”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Resmetirom: An Orally Administered, Smallmolecule, Liver-directed, β-selective THR Agonist for the Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis.[touchREV Endocrinol. 2023]Review Resmetirom: An Orally Administered, Smallmolecule, Liver-directed, β-selective THR Agonist for the Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis.Karim G, Bansal MB. touchREV Endocrinol. 2023 May; 19(1):60-70. Epub 2023 May 1.
- Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis.[Aliment Pharmacol Ther. 2024]Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis.Harrison SA, Ratziu V, Anstee QM, Noureddin M, Sanyal AJ, Schattenberg JM, Bedossa P, Bashir MR, Schneider D, Taub R, et al. Aliment Pharmacol Ther. 2024 Jan; 59(1):51-63. Epub 2023 Oct 2.
- A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis.[N Engl J Med. 2024]A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis.Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, et al. N Engl J Med. 2024 Feb 8; 390(6):497-509.
- Activation of thyroid hormone receptor-β improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis.[Br J Pharmacol. 2021]Activation of thyroid hormone receptor-β improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis.Kannt A, Wohlfart P, Madsen AN, Veidal SS, Feigh M, Schmoll D. Br J Pharmacol. 2021 Jun; 178(12):2412-2423. Epub 2021 Apr 6.
- Review The first MASH drug therapy on the horizon: Current perspectives of resmetirom.[Liver Int. 2024]Review The first MASH drug therapy on the horizon: Current perspectives of resmetirom.Petta S, Targher G, Romeo S, Pajvani UB, Zheng MH, Aghemo A, Valenti LVC. Liver Int. 2024 Apr 5; . Epub 2024 Apr 5.
- Resmetirom - LiverToxResmetirom - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...