NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ramelteon is a melatonin receptor agonist that is used for the treatment of insomnia. Ramelteon has not been implicated in causing serum enzyme elevations or clinically apparent liver injury.
Background
Ramelteon (ra mel' tee on) is a synthetic melatonin receptor agonist with affinity for both the melatonin type 1 and type 2 receptors (MT1 and MT2). These receptors are believed to be involved in the maintenance of the circadian rhythm that regulates the normal sleep-wake cycle. Melatonin itself has been proposed as therapy of sleep disturbances including insomnia and jet lag, but systematic reviews and meta analyses of controlled trials of various melatonin formulations have failed to demonstrate consistent efficacy. In contrast, ramelteon was found to reduce the average latency to persistent sleep with little residual sleepiness the following day or rebound insomnia upon withdrawal. Ramelteon was approved for use in chronic insomnia in the United States in 2005. It is available in 8 mg tablets under the brand name Rozerem. The recommended dose is 8 mg taken orally 30 minutes before going to bed. Side effects are few but may include daytime somnolence, fatigue, dizziness and headache and rarely hypersensitivity reactions with angioedema of the tongue and larynx.
Hepatotoxicity
In several clinical trials, ramelteon was found to be well tolerated and not associated with serum enzyme elevations or evidence of liver injury. Despite, wide scale use, it has not been convincingly linked to instances of clinically apparent liver injury. A single report of worsening liver disease with jaundice, ascites, bacterial peritonitis and death in a patient with alcoholic liver disease who had started ramelteon therapy a month before has been reported. Ramelteon is not recommended in patients with impaired hepatic function.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
Rameltoeon is a synthetic melatonin receptor agonist and is unrelated to melatonin structurally. It is unclear how ramelteon might cause liver injury, but it undergoes extensive first pass uptake by the liver and is metabolized by the cytochrome P450 system (largely CYP 1A2). For these reasons, its metabolism might be altered in persons with liver disease or alcoholism as well as by strong inducers or inhibitors of CYP enzymes.
Outcome and Management
Ramelteon has not been convincingly associated with serum enzyme elevations during therapy or with clinically apparent liver injury. However, persons with preexisting liver disease should either avoid use of ramelteon or be monitored for worsening of liver test results during therapy.
Drug Class: Sedatives and Hypnotics
Other Drugs in the Subclass, Melatonin and its Analogues: Melatonin, Tasimelteon
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ramelteon – Rozerem®
DRUG CLASS
Sedatives and Hypnotics
Product labeling at DailyMed, National Library of Medicine, NIH
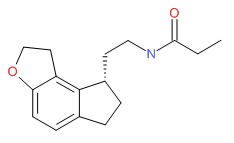
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ramelteon | 196597-26-9 | C16-H21-N-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 21 May 2018
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 731-4.(Expert review of hepatotoxicity published in 1999; melatonin and ramelteon are not discussed).
- Larrey D, Ripault MP. Anxiolytic agents. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 455-6.(Review of hepatotoxicity of hypnotics and sedatives discusses benzodiazepines, buspirone and valerian all of which have been linked to rare cases of liver injury; melatonin and ramelteon are not discussed).
- Mihic SJ, Harris RA. Hypnotics and sedatives. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 457-80.(Textbook of pharmacology and therapeutics).
- Penev PD, Zee PC. Melatonin: a clinical perspective. Ann Neurol 1997; 42: 545-53. [PubMed: 9382465](Discussion of physiological functions of melatonin and potential clinical uses).
- Seabra ML, Bignotto M, Pinto LR Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res 2000; 29: 193-200. [PubMed: 11068941](Controlled trial of melatonin [10 mg daily] vs placebo for 28 days in 40 volunteers found no changes or abnormalities of serum bilirubin, ALT, AST or Alk P during the 4 weeks of treatment).
- Ramelteon(Rozerem) for insomnia. Med Lett Drugs Ther 2005; 47 (1221): 89-91. [PubMed: 16267494](Concise review of the mechanism of action, efficacy and safety of ramelteon published shortly after its approval for use in insomnia in the US; adverse effects include somnolence, dizziness, nausea, fatigue, headache and insomnia, but no mention is made of ALT elevations or hepatotoxicity; mentions that melatonin itself is available in the US only as a "dietary supplement").
- Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ 2006; 332: 385-93. [PMC free article: PMC1370968] [PubMed: 16473858](Systematic review of literature on efficacy and safety of melatonin for sleep disorders; in 9 randomized controlled trials there was no overall evidence of benefit; in 10 studies evaluating safety there was no evidence of adverse effects occurring more often than with placebo, with use limited to 3 months).
- Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med 2006; 7: 17-24. [PubMed: 16309958](Controlled crossover trial of 4 doses of ramelteon vs placebo in 107 patients with chronic primary insomnia found no difference in rate of side effects compared to placebo, the most common being headache and somnolence; serum enzymes not tested).
- Devi V, Shankar PK. Ramelteon: a melatonin receptor agonist for the treatment of insomnia. J Postgrad Med 2008; 54: 45-8. [PubMed: 18296808](Review of the mechanism of action, efficacy and safety of ramelteon in treatment of insomnia; adverse events include headache, somnolence, dizziness, fatigue, insomnia and nausea; no mention made of ALT elevations or hepatotoxicity; superpharmacological, high doses of ramelteon cause liver cancer in mice).
- Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep 2009; 32: 351-60. [PMC free article: PMC2647789] [PubMed: 19294955](Controlled trial of 6 month course of ramelteon vs placebo in 451 adults with primary chronic insomnia; adverse effects were similar between the two groups; no mention of ALT levels).
- Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol 2012; 349: 91-104. [PMC free article: PMC3242827] [PubMed: 21939733](Review of the biologic basis of circadian rhythm including the role of melatonin).
- Drugs for insomnia. Treat Guidel Med Lett 2012; 10 (119): 57-60. [PubMed: 22777275](Guidelines for therapy of insomnia; mentions that benzodiazepine receptor agonists such as zaleplon, benzodiazepines, ramelteon and low doses of doxepin are effective and generally safe; the discussion of adverse events makes no mention of ALT elevations or hepatotoxicity of any of the recommended agents).
- Fourman LT, Robert Meyer B. Autoimmune hepatitis in association with ramelteon. J Clin Gastroenterol 2013; 47: 651-4. [PubMed: 23632362](50 year old man with chronic alcoholism developed worsening liver tests a month after starting ramelteon with subsequent jaundice, liver failure, bacterial peritonitis and death [bilirubin rising from 1.6 to a peak of 34.2 mg/dL, ALT 63 to 718 U/L, AST 57 to 858 U/L, and Alk P 105 to 273 U/L], a biopsy suggesting fulminant hepatitis and confluent necosis rather than worsening alcoholic hepatitis and cirrhosis).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to ramelteon or other sedatives or sleeping aids).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 82 [9%] were attributed to agents active in the central nervous system, but none were due to ramelteon or other sedatives or sleeping aids).
- Drugs for insomnia. Med Lett Drugs Ther 2015; 57 (1472): 95-8. [PubMed: 26147892](Concise review of the mechanism of action, efficacy, safety and costs of drugs for insomnia including ramelteon does not mention ALT elevations or hepatotoxicity due to any of the agents discussed).
- Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, Vollmer KA, Mergenhagen KA. Review of safety and efficacy of sleep medicines in older adults. Clin Ther 2016; 38: 2340-72. [PubMed: 27751669](Systematic review of the literature on the pharmacokinetics, clinically efficacy and safety of sleep medications in the elderly [above 60 years], mentions that ramelteon demonstrates similar efficacy and tolerance in the elderly to that in younger subjects; no mention of ALT elevations or hepatotoxicity).
- Mahableshwarkar AR, Calabrese JR, Macek TA, Budur K, Adefuye A, Dong X, Hanson E, et al. Efficacy and safety of sublingual ramelteon as an adjunctive therapy in the maintenance treatment of bipolar I disorder in adults: A phase 3, randomized controlled trial. J Affect Disord 2017; 221: 275-82. [PubMed: 28662460](Among 642 patients with bipolar disorder treated with one of 3 doses of ramelteon or placebo sublingually once daily at bedtime, rates and timing of relapse were the same in all four groups as were rates and types of adverse events; no mention of ALT elevations or hepatotoxicity).
- Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production.[Respir Res. 2020]Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production.Wu GC, Peng CK, Liao WI, Pao HP, Huang KL, Chu SJ. Respir Res. 2020 Mar 6; 21(1):65. Epub 2020 Mar 6.
- Review Ramelteon for the treatment of insomnia.[Clin Ther. 2006]Review Ramelteon for the treatment of insomnia.Borja NL, Daniel KL. Clin Ther. 2006 Oct; 28(10):1540-55.
- Melatonin Receptor Agonist Ramelteon Reduces Ischemia-Reperfusion Injury Through Activation of Mitochondrial Potassium Channels.[J Cardiovasc Pharmacol. 2018]Melatonin Receptor Agonist Ramelteon Reduces Ischemia-Reperfusion Injury Through Activation of Mitochondrial Potassium Channels.Stroethoff M, Christoph I, Behmenburg F, Raupach A, Bunte S, Senpolat S, Heinen A, Hollmann MW, Mathes A, Huhn R. J Cardiovasc Pharmacol. 2018 Aug; 72(2):106-111.
- Review Searching for new options for treating insomnia: are melatonin and ramelteon beneficial?[J Psychiatr Pract. 2006]Review Searching for new options for treating insomnia: are melatonin and ramelteon beneficial?Bellon A. J Psychiatr Pract. 2006 Jul; 12(4):229-43.
- Pharmacotherapy of insomnia with ramelteon: safety, efficacy and clinical applications.[J Cent Nerv Syst Dis. 2011]Pharmacotherapy of insomnia with ramelteon: safety, efficacy and clinical applications.Pandi-Perumal SR, Spence DW, Verster JC, Srinivasan V, Brown GM, Cardinali DP, Hardeland R. J Cent Nerv Syst Dis. 2011; 3:51-65. Epub 2011 Apr 12.
- Ramelteon - LiverToxRamelteon - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...