NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Quinine is a natural cinchona alkaloid that has been used for centuries in the prevention and therapy of malaria. Quinine is also used for idiopathic muscle cramps. Quinine therapy has been associated with rare instances of hypersensitivity reactions which can be accompanied by hepatitis and mild jaundice.
Background
Quinine (kwye' nine) is the major alkaloid contained in the powered bark of the South American cinchona tree. The powder of the cinchona tree bark was used by native Quechna Indians in Peru to treat fever and was introduced into Western medicine when Jesuit priests took samples to Europe. The major effect of the cinchona tree bark was against malaria. Quinine acts against the asexual erythrocytic forms of malaria, including Plasmodium vivax, malariae and falciparum and is gametosidal to P. vivax and malariae. The use of quinine for malaria has been largely replaced by chloroquine, which is more potent and better tolerated. However, quinine is still used intravenously in some instances of drug resistant P. falciparum. In addition, quinine has been used frequently for nocturnal leg cramps, although the data in support of its efficacy is controversial, and quinine by prescription is no longer approved for this use. Quinine is available in multiple generic forms in low doses as an over-the-counter medication and as tablets of 324 mg for therapy of malaria. The use and dosage for malaria treatment and prevention requires higher doses than are used for leg cramps. Regularly updated recommendations on the therapy of malaria including specific details on diagnosis, management, drug dosage and safety are available at the CDC website: http://www.cdc.gov/malaria/. Common side effects of quinine include headache, dizziness, blurred vision, gastrointestinal upset, thrombocytopenia and hypersensitivity reactions.
Hepatotoxicity
There is little evidence that chronic quinine therapy is associated with elevations in serum enzymes, although it has not been carefully assessed. However, there have been several reports of acute hypersensitivity reactions to quinine that include hepatic involvement. The reactions usually arise after 1 to 2 weeks of therapy, but can appear within 24 hours of restarting quinine or with rechallenge. The clinical features are marked by fatigue, nausea, vomiting, diffuse muscle aches, arthralgias and high fever. Blood testing at an early stage shows increases in serum aminotransferase and alkaline phosphatase levels as well as mild jaundice, which can deepen for a few days even after stopping quinine. The pattern of serum enzymes elevations is typically cholestatic or mixed. Rash is uncommon and eosinophilia is not typical, despite the presence of other signs of hypersensitivity (fever, arthralgias). Autoantibodies are not typically found. Liver biopsies usually show mild injury and small epithelioid granulomas, as are typically found in many organs during systemic hypersensitivity reactions. A similar clinical signature of liver injury occurs with quinidine, an optical isomer of quinine that is used predominantly as an antiarrhythmic.
Likelihood score: B (highly likely cause of clinically apparent liver injury).
Mechanism of Injury
The hepatotoxicity of quinine is clearly due to a hypersensitivity reaction and there is no evidence for a direct hepatotoxic effect. There is likely to be a genetic predisposition to this hypersensitivity.
Outcome and Management
The hepatotoxicity of quinine is usually mild and resolves within 1 to 4 weeks of stopping. In many instances, jaundice and liver test abnormalities may worsen for a few days after stopping quinine, but fatalities have not been reported, and recovery is usually rapid. Because of the rapidity of recovery, therapy with corticosteroids is best avoided. There is cross reactivity to quinidine (the optical isomer of quinine which is used as an antiarrhythmic agent) and other exposures to quinine should be avoided. Because quinine is in tonic water as well as many over-the-counter medications, medicinal foods and herbal preparations, patients with quinine sensitivity should make sure that they are not inadvertently exposed.
Drug Class: Antimalarial Agents
CASE REPORT
Case 1. Acute hypersensitivity and cholestatic jaundice due to quinine.
[Modified from: Farver DK, Lavin MN. Quinine-induced hepatotoxicity. Ann Pharmacother 1999: 33: 32-4. PubMed Citation]
A 57 year old woman was given quinine for nocturnal leg cramps and developed high fever, chills, fatigue, muscle aches and nausea necessitating hospital admission within 24 hours of starting quinine. She had a history of hypertension, diabetes, hyperlipidemia and coronary artery disease for which she was taking insulin, lisinopril, amlodipine, aspirin, furosemide and multivitamins. She used acetaminophen occasionally. She did not drink alcohol and had no risk factors for acquiring viral hepatitis. On examination, she was febrile (39.5 oC), but without rash, lymphoadenopathy or signs of acute or chronic liver disease. Laboratory testing showed normal blood counts, but elevations in alkaline phosphatase and aminotransferase levels without hyperbilirubinemia (Table). Tests for hepatitis A and B were negative as were autoantibodies. Her serum enzymes remained elevated and several days later she became jaundiced. Endoscopic retrograde cholangiopancreatography showed that the biliary tree was normal and the gallbladder was without gallstones. Quinine had been continued during the hospitalization, but was stopped on day 6, whereupon her symptoms began to improve. Hyperbilirubinemia resolved within a few days of stopping quinine and serum enzymes were normal or near normal 2 weeks later.
Key Points
| Medication: | Quinine |
| Pattern: | Cholestatic (R=2.0→1.2) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 1 day |
| Recovery: | 3 weeks |
| Other medications: | Insulin, lisinopril, amlodipine, aspirin, furosemide, and multiple vitamins chronically, occasional acetaminophen |
Laboratory Values
Comment
A dramatic hypersensitivity reaction with jaundice, following a single dose of quinine in a patient without a known history of previous exposure. Improvement was prompt once quinine was stopped.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Quinine – Generic, Qualaquin®
DRUG CLASS
Antimalarial Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
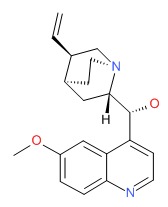
| Quinine | 130-95-0 | C20-H24-N2-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 15 May 2018
- Zimmerman HJ. Antiprotozoal agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 623-5.(Expert review of hepatotoxicity published in 1999; mentions that quinine is an ancient drug that has been incriminated in rare instances of granulomatous hepatitis).
- Vinetz JM, Clain J, Bounkeua V, Eastman RT, Fidock D. Chemotherapy of malaria. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1383-418.(Textbook of pharmacology and therapeutics).
- Colding H. Et tilfaelde af kinidinallergi med feber og leverpavirkning. [A case of quinine-allergy with fever and liver injury]. Ugeskr Laeger 1969; 131: 1657-8. [PubMed: 5365434](74 year old woman developed fever and fatigue 2-3 weeks after starting quidinine with rise in bilirubin [0.4 to 2.0 mg/dL], AST [0.7 to 7.3 units] and Alk P [2.2 to 10.5 units] and resolution within 1 week of stopping; positive rechallenge).
- Deisseroth A, Morganroth J, Winokour S. Quinidine-induced liver disease. Ann Intern Med 1972; 77: 595-7. [PubMed: 4642741](77 year old developed fever 11 days after starting quinidine followed by increases in ALT to ~210 U/L and LDH to 310 U/L, with prompt improvement upon stopping and recurrence of fever and ALT elevations within 24 hours of rechallenge).
- Winkler JW. Quinidine hepatotoxicity? Ann Intern Med 1973; 78: 460. [PubMed: 4694908](Letter in response to Deisseroth et al. [1972] raising the issue of role of dioctyl calcium sulfosuccinate [Surfak] in the hepatic injury; authors reply that this was unlikely).
- Murphy RJ, Rymer W. Quinidine induced liver disease? Ann Intern Med 1973; 78: 460. [PubMed: 4711794](62 year old man with 3 episodes of fever which in retrospect was thought to be due to quinidine with AST elevations of ~135 U/L, given challenge dose and developed fever to 39.4 oC but no change in AST).
- Chajek T, Lehrer B, Geltner D, Levij IS. Quinidine-induced granulomatous hepatitis. Ann Intern Med 1974; 81: 774-6. [PubMed: 4433082](69 year old man developed fever 7 days after starting quinidine [bilirubin 1.0 mg/dL, Alk P 205 U/L, AST 430 U/L] resolving within 1 week; rechallenge led to fever within 24 hours and rise of AST to 250 U/L, Alk P 305 U/L, biopsy showing granulomas, repeat rechallenge showing the same with biopsy before and after).
- Chajek T. Quinidine and granulomatous hepatitis. Ann Intern Med 1975; 82: 282. [PubMed: 1115455](After 1974 article, saw another case: 64 year old man developed fever 12 days after starting quinidine with Alk P and AST elevations, with rapid improvement on stopping and recurrence on restarting, biopsy showing granulomas).
- Handler SD, Hirsch NR, Hass K, Davidson FZ. Quinidine hepatitis. Arch Intern Med 1975; 135: 871-2. [PubMed: 48362](72 year old woman developed abnormal liver tests [AST 367 U/L] 7 months after starting quinidine, remaining elevated for 9 months and AST rising to 800 U/L [no jaundice and Alk P peak 110 U/L], rapid improvement with stopping and positive rechallenge to one dose with symptoms and AST rising to 110 U/L).
- Koch MJ, Seeff LB, Crumley CE, Rabin L, Burns WA. Quinidine hepatitis: a report of a case and review of the literature. Gastroenterology 1976; 70: 1136-40. [PubMed: 1269875](47 year old man developed fever and abdominal pain 10 days after starting quinidine [normal bilirubin, peak ALT 910 U/L, Alk K 210 U/L], resolving rapidly when quinidine was stopped, and with recurrence of symptoms and mild ALT elevation within days of rechallenge).
- Geltner D, Chajek T, Rubinger D, Levij IS. Quinidine hypersensitivity and liver involvement: a survey of 32 patients. Gastroenterology 1976; 70: 650-2. [PubMed: 1261756](Among 487 patients who received quinidine over a 4 year period, 32 [6.5%] developed a hypersensitivity reaction including 10 [2%] with liver involvement with fever, Alk P and AST elevations and jaundice in half, usually after 4-14 days of therapy, resolving rapidly, liver biopsies showing granulomas and focal necrosis).
- Dzur JR. Letter: Quinidine hepatotoxicity. JAMA 1976; 235: 908. [PubMed: 946110](Patient developed jaundice one month after starting quinidine, resolving with stopping; no details given).
- Rotmensch HH, Rubinstein A, Livni E, Liron M, Ilie B. [Quinidine-induced subclinical hepatitis]. Harefuah. 1980; 98: 211-2. Hebrew. [PubMed: 6997146](68 year old man developed fever and arthralgias within weeks of starting quinidine, liver tests were normal but liver biopsy showed small granulomas and spotty hepatocyte necrosis; fever resolved within 7 days of stopping quinidine).
- Bramlet DA, Posalaky Z, Olson R. Granulomatous hepatitis as a manifestation of quinidine hypersensitivity. Arch Intern Med 1980; 140: 395-7. [PubMed: 7362358](64 year old woman developed fever and fatigue one month after starting quinidine [bilirubin 1.8 mg/dL, ALT 327 U/L, Alk P 105 U/L], rapid resolution and positive rechallenge in one day with fever and AST elevation, underwent 3 liver biopsies showing presence, disappearance and reappearance of small granulomas).
- Tiliakos N, Waites TF. Multiform quinidine toxicity. South Med J 1981; 74: 1267-8. [PubMed: 7197395](39 year old man developed fever and fatigue 8 days after starting quinidine with rash [4% eosinophils, thrombocytopenia, bilirubin 4.0 mg/dL, AST 280, Alk P 150 U/L], with rapid improvement on stopping quinidine).
- Slezak P. Quinidine hepatotoxicity. Med J Aust 1981; 1: 139. [PubMed: 7219288](58 year old man developed fever, fatigue and abdominal pain 10 days after starting quinidine, followed by jaundice [bilirubin 1.6 mg/dL, ALT 208 U/L, Alk P 1.5 times ULN]).
- Karoly R, Ferenc S. [An unusual side effect of quinidine: liver damage, pneumonia]. Orv Hetil 1983; 124: 055-6. Hungarian. [PubMed: 6634153](59 year old man developed fever, liver injury and pneumonitis within days of starting quinidine [bilirubin 3.1 mg/dL, ALT 120 U/L, Alk P 183 U/L, 6% eosinophils]).
- Urdahl P, Bjørkheim A. [Quinidine-induced hepatitis. A case report and review of the literature]. Tidsskr Nor Laegeforen 1983; 103: 760-3. Norwegian. [PubMed: 6879565](60 year old woman developed fever and fatigue within a week of starting quinidine [bilirubin 2.7 mg/dL, ALT 292 U/L, Alk P 229 U/L], with rapid recovery and positive rechallenge).
- Katz B, Weetch M, Chopra S. Quinine-induced granulomatous hepatitis. Br Med J 1983; 286: 264-5. [PMC free article: PMC1546488] [PubMed: 6402064](65 year old woman with episodic fever, nausea and polyarthralgias during 5 months of intermittent quinine use for leg cramps, [bilirubin 0.5 mg/dL, ALT 460 U/L, Alk P 375 U/L], biopsy showing granulomas; positive rechallenge with fever and ALT to 480 U/L).
- Nirodi NS. Quinine induced granulomatous hepatitis. Br Med J(Clin Res Ed) 1983; 286: 647. [PMC free article: PMC1546846] [PubMed: 6402190](Letter questioning the presence of granulomas in case of Katz et al. [1983]).
- Smally AJ. When to leave well enough alone—two cases. Hosp Pract 1985; 20: 48-9. [PubMed: 3918052](Two cases of quinidine hepatotoxicity; first case with nausea and fever 10 days after starting quinidine followed by jaundice [bilirubin 5.4 mg/dL, AST 168 U/L, 3-4% eosinophils], and rapid recovery upon stopping; second with onset of fever, fatigue and headache 3 weeks after starting quinidine [ALT 121 U/L], rapid resolution upon stopping).
- Tanaka N, Matsushita E, Morimoto H, Kobayashi K, Hattori N. [Toxic hepatitis induced by cardiovascular agents]. Nippon Rinsho 1985; 43: 1172-5. Japanese. [PubMed: 3900470](Case series of drug induced liver disease due to cardiovascular agents, including one case due to quinidine).
- Knobler H, Levij IS, Gavish D, Chajek-Shaul T. Quinidine-induced hepatitis: a common and reversible hypersensitivity reaction. Arch Intern Med 1986; 146: 526-8. [PubMed: 3954525](Among 90 cases of drug induced liver injury seen over 10 years in one referral hospital in Israel, 33 were due to quinidine [2% of recipients] with typical signature of fever, arising within first 4 weeks of therapy, both Alk P and ALT modestly elevated, only 6 with jaundice, none fulminant, all resolved rapidly with no long term consequences; 8 rechallenged and all redeveloped fever, 6 had Alk P and AST elevations as well).
- Zatuchni J. Quinidine-induced hepatitis. Arch Intern Med 1986; 146: 2077, 2081. [PubMed: 3767557](Letter questioning Knobler et al. [1986] why quinidine was used so much in view of its questionable efficacy; reply by authors).
- Pariente EA, Maitre F, Marchand JP. [Hepatitis caused by quinidine. Study of a case and review of the literature]. Gastroenterol Clin Biol 1986; 10: 255-8. French. [PubMed: 3732735](63 year old woman developed fatigue and jaundice 1 month after starting quinidine [bilirubin 2.2 mg/dL, ALT 109 U/L, Alk P 684 U/L], biopsy showing granulomas, rapid recovery but still had hepatomegaly and elevated Alk P in follow up).
- Bourlière M, Bernuau J, Rueff B, Benhamou JP. Quinidine phenylethyl-barbiturate-induced fulminant hepatitis in a pregnant woman. A case report. J Hepatol 1988; 6: 214-6. [PubMed: 3411101](19 year old pregnant woman developed rash and fever one month after starting quinidine and barbiturate [bilirubin 1.6 rising to 18.8 mg/dL, ALT 296 to 800 U/L, Alk P 267 U/L, prothrombin index 13%], delivering normal baby, but subsequently developing hepatic failure and undergoing successful liver transplant).
- Alix M, Mosquet B, Adrien A, Cuny G, Raffy P, Moulin M. [Cholestatic hepatitis caused by quinidine phenylethylbarbiturate, and sarcoidosis. Apropos of a case]. Therapie 1989; 44: 69. French. [PubMed: 2734725](68 year old developed jaundice 2 months after starting quinidine and a barbiturate with mild fever [bilirubin 15 mg/dL, ALT 200 U/L, Alk P 342 U/L], which improved with stopping, but enzymes remained high and follow up liver biopsy suggested sarcoidosis).
- Mathur S, Dooley J, Scheuer PJ. Quinine induced granulomatous hepatitis and vasculitis. BMJ 1990; 300: 613. [PMC free article: PMC1662381] [PubMed: 2108777](67 year old man with fever, rash and polyarthralgias after 2 months of intermittent quinine therapy for leg cramps [bilirubin 0.6 mg/dL, AST 100 U/L, Alk P 1668 U/L], granulomas on biopsy and rapid recovery upon stopping).
- Punukollu RC, Kumar S, Mullen KD. Quinine hepatotoxicity: an under recognized or rare phenomenon? Arch Intern Med 1990; 150: 1112-3. [PubMed: 2331190](37 year old woman developed fever, headache, arthralgias and abdominal pain without rash 2-3 weeks after starting quinine for leg cramps [bilirubin 1.8 mg/dL, ALT 128 U/L, Alk P 327 U/L, eosinophils 6%], rapid improvement with stopping quinine, normal in 1 week; positive rechallenge to one dose with fever and ALT to 64 U/L).
- Perez JA, Stryker J, Arsura EL, Hewitt JM. Probable quinine-induced hepatotoxicity. West J Med 1994; 160: 59-60. [PMC free article: PMC1022263] [PubMed: 8128710](74 year old man developed fever, abdominal pain and jaundice one month after starting quinine for leg cramps [bilirubin 7.2 mg/dL, ALT 241 U/L, Alk P 314 U/L, ESR 65], biopsy showing granulomas and resolving rapidly upon stopping).
- Man-Song-Hing M, Wells G. Meta-analysis of efficacy of quinine for treatment of nocturnal leg cramps in elderly people. BMJ 1995; 10: 13-7. [PMC free article: PMC2548434] [PubMed: 7827545](Meta analysis of 107 patients from 6 clinical trials; quinine reduced number of nights without leg cramps by 27%).
- Horney E, Lagerstedt C, Wadenvik H. [Quinine induced thrombocytopenia and granulomatous hepatitis]. Lakartidningen 1996; 93: 361-4. Swedish. [PubMed: 8628066](58 year old man developed intermittent fevers [normal bilirubin, ALT 185 U/L, GGT 145 U/L, thrombocytopenia]; positive rechallenge).
- Hou M, Horney E, Stockelberg D, Jacobsson S, Kutti J, Wadenvik H. Multiple quinine-dependent antibodies in a patient with episodic thrombocytopenia, neutropenia, lymphocytopenia, and granulomatous hepatitis. Blood 1997; 90: 4806-11. [PubMed: 9389697](58 year old man with recurrent, transient bouts of fever, nausea, thrombocytopenia and ALT elevations [4-6 times ULN] shown to be due to intermittent exposure to quinine, with positive rechallenge and demonstration of quinine dependent antibodies to platelets and white blood cells).
- Farver DK, Lavin MN. Quinine-induced hepatotoxicity. Ann Pharmacother 1999: 33: 32-4. [PubMed: 9972382](57 year old woman developed fever, nausea and myalgias within 24 hours of starting quinine for leg cramps with temperature 39.5 oC [bilirubin 0.7 mg/dL, ALT 184 U/L, Alk P 192 U/L], resolving in 3 weeks).
- Howard MA, Hibbard AB, Terrell DR, Medina PJ, Vesely SK, George JN. Quinine allergy causing acute severe systemic illness: report of 4 patients manifesting multiple hematologic, renal, and hepatic abnormalities. Proc (Bayl Univ Med Cent) 2003; 16: 21-6. [PMC free article: PMC1200805] [PubMed: 16278718](Four case reports of quinine induced thrombocytopenia with other systemic manifestations, including prominent AST [131, 992, 741 and 3735 U/L] and mild Alk P [74-170 U/L] elevations and 2 with jaundice [bilirubin 4.3 and 16.1 mg/dL]; often inadvertent quinine use from over-the-counter pills).
- Schlegel A. Factitious granulomatous hepatitis? Am J Med 2004; 116: 500-1. [PubMed: 15047046](35 year old woman with relapsing episodes of fever, abdominal pain and ALT elevations [1488 U/L] and biopsy showing small granulomas, finally confessed to intermittent, purposeful use of quinine).
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004; 27: 25-61. [PubMed: 14720085](Review of toxicities and safety of antimalarials: quinine can cause a characteristic hypersensitivity reaction with liver injury).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. (Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, no cases were attributed to. [PMC free article: PMC4446235] [PubMed: 25754159]quinine or quinidine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Historical chemical annotations of Cinchona bark collections are comparable to results from current day high-pressure liquid chromatography technologies.[J Ethnopharmacol. 2020]Historical chemical annotations of Cinchona bark collections are comparable to results from current day high-pressure liquid chromatography technologies.Canales NA, Gress Hansen TN, Cornett C, Walker K, Driver F, Antonelli A, Maldonado C, Nesbitt M, Barnes CJ, Rønsted N. J Ethnopharmacol. 2020 Mar 1; 249:112375. Epub 2019 Nov 4.
- Review Are there alternatives to the use of quinine to treat nocturnal leg cramps?[Consult Pharm. 2008]Review Are there alternatives to the use of quinine to treat nocturnal leg cramps?Guay DR. Consult Pharm. 2008 Feb; 23(2):141-56.
- On the history of Cinchona bark in the treatment of Malaria.[Dan Medicinhist Arbog. 2016]On the history of Cinchona bark in the treatment of Malaria.Permin H, Norn S, Kruse E, Kruse PR. Dan Medicinhist Arbog. 2016; 44:9-30.
- Quinine for cramps.[Aust Fam Physician. 1998]Quinine for cramps.Pinn G. Aust Fam Physician. 1998 Oct; 27(10):922-3.
- Review Should people with nocturnal leg cramps drink tonic water and bitter lemon?[Psychol Rep. 1999]Review Should people with nocturnal leg cramps drink tonic water and bitter lemon?Brasić JR. Psychol Rep. 1999 Apr; 84(2):355-67.
- Quinine - LiverToxQuinine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...