NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pretomanid is a nitroimidazooxazine antimycobacterial agent used in combination with other antituberculosis drugs in the treatment of multidrug resistant tuberculosis. The addition of pretomanid to antituberculosis drug regimens has been linked to an increased rate of transient serum liver test abnormalities during treatment and to several instances of mild, clinically apparent liver injury.
Background
Pretomanid (pre toe’ ma nid) is a unique antimycobacterial agent that has activity against multidrug resistant mycobacterium tuberculosis both in vitro and in vivo and was first approved for use in the United States in 2019. A nitroimidazooxazine, pretomanid is believed to act via inhibition of mycolic acid synthesis, blocking mycobacterial cell wall synthesis. Its current indications are for multidrug resistant pulmonary tuberculosis in combination with other antituberculosis agents. It is not approved for use in latent or drug sensitive tuberculosis or in atypical mycobacterial infections. In addition, pretomanid has not been shown to be effective for extra-pulmonary tuberculosis. Pretomanid is available in tablets of 200 mg under the brand name Pretomanid. The recommended dose is 200 mg once daily in combination with bedaquiline and linezolid for 26 weeks. Side effects of this regimen are common and include peripheral neuropathy, acne, anemia, nausea, vomiting, dyspepsia, anorexia, weight loss, diarrhea, abdominal pain, headache, rash, pruritus, cough, pleuritic pain, hemoptysis, back pain, visual impairment and hypoglycemia. Laboratory abnormalities include anemia and increases in serum aminotransferase levels and amylase. Rare, but potentially serious adverse reactions include myelosuppression, peripheral and optic neuropathy, prolongation of the QTc interval, lactic acidosis and reproductive effects including impaired fertility.
The management of multidrug resistant tuberculosis is challenging and should be under the direction of physicians with expertise in tuberculosis therapy and management of its side effects. Optimal regimens of therapy for tuberculosis are complex and change frequently. Regularly updated recommendations on use of pretomanid and other drugs for tuberculosis, including indications, contraindications, warnings, dosages and monitoring recommendations are available at the Centers for Disease Control and Prevention website: http://www.cdc.gov/tb/publications/guidelines/Treatment.htm.
Hepatotoxicity
Liver test abnormalities occur in 30% of patients treated with multiple drug regimens that include pretomanid. These abnormalities are usually asymptomatic, mild-to-moderate in severity and self-limited in duration. In many instances, it is difficult to determine which of the antituberculosis medications account for the abnormalities, but regular monitoring of liver tests is recommended during triple therapy with pretomanid, bedaquiline and linezolid. Clinically apparent liver injury has been reported with pretomanid-based therapies, but largely in regimens that included moxifloxacin or pyrazinamide or both. The clinical features, course and outcome of these cases has not been well defined. In the pivotal study of pretomanid combined with bedaquiline and linezolid in 109 adults with drug resistant pulmonary tuberculosis, 12 patients (11%) developed ALT levels above 3 times the upper limit of normal (ULN) of whom two also developed mild jaundice (bilirubin above twice but less than 3 times ULN) arising during month two of therapy. Both patients had mild nausea accompanying the liver test abnormalities, and the abnormalities resolved in both with temporary interruption in treatment, followed by re-initiation using lower dose linezolid without change in the pretomanid dose. In this pivotal trial, most adverse events were attributed to linezolid.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which pretomanid might cause serum aminotransferase elevations or clinically apparent liver injury is not known but is likely due to production of a toxic intermediate during its metabolism. Pretomanid is metabolized by the liver via multiple reductive and oxidative pathways, the P450 system (predominantly CPY 3A4) accounting for approximately 20% of its metabolism. Nevertheless, pretomanid is susceptible to drug-drug interactions with agents that induce or inhibit CYP3A4 and with substrates of the organic anion transporter 3 (OAT-3) which also participates it is metabolism and excretion.
Outcome and Management
Patients on pretomanid should be monitored with liver tests, including serum bilirubin, ALT, AST and alkaline phosphatase before starting therapy and at intervals thereafter. Pretomanid should be discontinued for persistent increases in liver test abnormalities, ALT elevations accompanied by increases in total bilirubin of more than twice normal or by any symptom or sign of liver injury. There is no evidence to suggest cross sensitivity to liver injury or adverse events between pretomanid and other antituberculosis medications.
Drug Class: Antituberculosis Agents
Other Drugs in the Class: Bedaquline, Capreomycin, Cycloserine, Ethambutol, Ethionamide, Isoniazid, Pyrazinamide, Rifabutin, Rifampin, Rifapentine, Streptomycin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pretomanid – Pretomanid®
DRUG CLASS
Antituberculosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
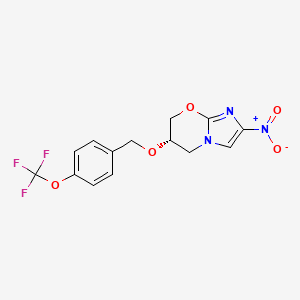
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pretomanid | 187235-37-6 | C14-H12-F3-N3-O5 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 04 November 2019
- Zimmerman HJ. Antituberculosis agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 611-21.(Extensive review of hepatotoxicity of antituberculosis medications published in 1999 before the availability of pretomanid).
- Verma S, Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 483-504.(Review of hepatotoxicity of antituberculosis drugs published before the availability of bedaquiline or pretomanid).
- Gumba T. Chemotherapy of tuberculosis, mycobacterium avium complex disease and leprosy. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1549-70.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/212862Orig1s000OtherR .pdf . (FDA website with review of safety and efficacy of pretomanid includes specific analysis of its potential hepatotoxicity [pages 31-49]). - Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob Agents Chemother. 2009;53:3720–5. [PMC free article: PMC2737845] [PubMed: 19528280](Among 58 healthy volunteers dose once daily with pretomanid [15 to 1500 mg] once daily for up to 7 days, there were “no significant or serious adverse events”; no mention of ALT or AST elevations).
- Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, van Niekerk C, et al. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob Agents Chemother. 2012;56:3027–31. [PMC free article: PMC3370777] [PubMed: 22430968](Among 69 South African adults with pulmonary tuberculosis enrolled in a dose-ranging 14-day study, response rates were similar with 100 and 200 mg daily and there were no drug related serious adverse events; no mention of ALT or AST elevations).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were linked to pretomanid).
- Diacon AH, Donald PR, Pym A, Grobusch M, Patientia RF, Mahanyele R, Bantubani N, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother. 2012;56:3271–6. [PMC free article: PMC3370813] [PubMed: 22391540](47 patients with multidrug resistant tuberculosis were treated with bedaquiline or placebo with background regimens for up to 6 months; major side effects not mentioned).
- Roehr B. Trial tests new combination of drugs to treat tuberculosis. BMJ. 2012;344:e2216. [PubMed: 22434129](News report that the Global Alliance for TB Drug Development had launched a trial of pretomanid [PA 824], a new drug candidate for drug resistant tuberculosis).
- Centers for Disease Control and Prevention. Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (Sirturo) for the treatment of multidrug-resistant tuberculosis. MMWR Recomm Rep. 2013 Oct 25;62(RR-09):1–12. [PubMed: 24157696](Recommendations on use of bedaquiline mentions that patients should be monitored monthly and bedaquiline should be discontinued for persistent ALT or AST elevations, concurrent elevations of serum bilirubin, ALT or AST elevations above 8 times the ULN, or any liver test abnormalities accompanied by symptoms of liver injury).
- Dooley KE, Luetkemeyer AF, Park JG, Allen R, Cramer Y, Murray S, Sutherland D, et al. AIDS Clinical Trials Group A5306 Study Team. Phase I safety, pharmacokinetics, and pharmacogenetics study of the antituberculosis drug PA-824 with concomitant lopinavir-ritonavir, efavirenz, or rifampin. Antimicrob Agents Chemother. 2014;58:5245–52. [PMC free article: PMC4135849] [PubMed: 24957823](Study of pharmacokinetics of pretomanid when combined with various antiretroviral agents demonstrated that maximal plasma levels were reduced when combined with efavirenz [-28%] or rifampin [-53%], but reductions were less [-13%] when combined with lopinavir-ritonavir).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 408 were attributed to antimicrobial agents including 52 to antituberculosis agents, but no cases were attributed to pretomanid or bedaquiline).
- Dawson R, Diacon AH, Everitt D, van Niekerk C, Donald PR, Burger DA, Schall R, et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet. 2015;385(9979):1738–47. [PubMed: 25795076](Among 207 adults with pulmonary tuberculosis treated with standard therapy or pretomanid in combination with moxifloxacin and pyrazinamide for 8 weeks, antimycobacterial activity was similar with all regimens and pretomanid regimens had excellent activity against multidrug resistant infections, while adverse event rates were similar including ALT or AST elevations above 3 times ULN [12%, 17% and 18% in the 3 pretomanid arms vs 12% in controls]).
- Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, Nardell EA, et al. Lancet Respiratory Medicine drug-resistant tuberculosis Commission group. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7:820–6. [PubMed: 31486393](Review of the epidemiology, pathogenesis, diagnosis and management of multidrug resistant tuberculosis; does not discuss hepatotoxicity of therapeutic drug regimens).
- Keam SJ. Pretomanid: First Approval. Drugs. 2019 Oct 3; [Epub ahead of print] [PubMed: 31583606](Summary of the US FDA approval of pretomanid combined with bedaquiline and linezolid as therapy of multidrug resistant tuberculosis for 26 weeks; mentions that common adverse events noted in the 109 patients treated in registration trials included peripheral neuropathy [81%], acne [39%], anemia [37%], nausea [37%], vomiting [34%], myalgia [29%], headache [28%], increased aminotransferase [28%] and GGT levels [17%], dyspepsia [24%], anorexia [22%], rash [21%], pruritus [20%] and abdominal pain [19%], but there were no liver related serious adverse events or deaths).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Pretomanid: First Approval.[Drugs. 2019]Review Pretomanid: First Approval.Keam SJ. Drugs. 2019 Nov; 79(16):1797-1803.
- Review Bedaquiline.[LiverTox: Clinical and Researc...]Review Bedaquiline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pretomanid: The latest USFDA-approved anti-tuberculosis drug.[Indian J Tuberc. 2021]Review Pretomanid: The latest USFDA-approved anti-tuberculosis drug.Deb U, Biswas S. Indian J Tuberc. 2021 Apr; 68(2):287-291. Epub 2020 Sep 6.
- Long-Term Effects on QT Prolongation of Pretomanid Alone and in Combinations in Patients with Tuberculosis.[Antimicrob Agents Chemother. 2...]Long-Term Effects on QT Prolongation of Pretomanid Alone and in Combinations in Patients with Tuberculosis.Li H, Salinger DH, Everitt D, Li M, Del Parigi A, Mendel C, Nedelman JR. Antimicrob Agents Chemother. 2019 Oct; 63(10). Epub 2019 Sep 23.
- Toxicity and toxicokinetic assessment of an anti-tubercular drug pretomanid in cynomolgus monkeys.[Toxicol Rep. 2022]Toxicity and toxicokinetic assessment of an anti-tubercular drug pretomanid in cynomolgus monkeys.Bruning-Barry R, Ambroso JL, Dillberger J, Yang TJ. Toxicol Rep. 2022; 9:927-936. Epub 2022 Apr 22.
- Pretomanid - LiverToxPretomanid - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...