NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pitolisant is a histamine type 3 receptor (H3) antagonist and inverse agonist that is used in the therapy of excessive daytime sleepiness and cataplexy in patients with narcolepsy. Pitolisant has not been associated with serum enzyme elevations during therapy or to instances of idiosyncratic acute liver injury.
Background
Pitolisant (pi tol’ i sant) is an orally available, small molecule histamine 3 (H3) receptor antagonist and inverse agonist that is used to treat excessive daytime sleepiness in adults with narcolepsy and for cataplexy associated with narcolepsy. Narcolepsy is associated with a deficiency of hypothalamic cells producing orexin, a neuropeptide that acts as an excitatory neurotransmitter on neurons that produce wakefulness. H3 receptors are found in the central nervous system where histamine acts as excitatory neurotransmitter promoting wakefulness. Pitolisant was found to increase wakefulness in animal models of narcolepsy and clinical trials demonstrated that it decreased excessive daytime sleepiness in patients with narcolepsy. It also decreased the frequency of episodes of cataplexy, a frequent complication of narcolepsy. Pitolisant was approved in the United States in 2019 as therapy for excessive daytime sleepiness in adults with narcolepsy and was subsequently also approved for treatment of cataplexy due to narcolepsy. Pitolisant is available in tablets of 4.45 and 17.8 mg under the brand name Wakix. The recommended maintenance dose in adults is 17.8 mg once daily after an initial titration period of one week of 8.9 mg daily. Side effects can include headache, insomnia, anxiety, dizziness, increase in appetite, weight gain, abdominal discomfort and nausea. Uncommon, but potentially serious side effects include prolongation of the QTc interval, and it is contraindicated in patients with bradycardia and should be used cautiously in patients receiving other medications that can prolong the QTc interval.
Hepatotoxicity
In placebo-controlled trials of pitolisant in patients with narcolepsy, minor serum aminotransferase elevations occurred in a small proportion of patients during therapy, but rates of enzyme elevations were similar to those in placebo recipients. In preregistration trials, there were no instances of clinically apparent liver injury or serum aminotransferase elevations with jaundice attributable to pitolisant. Since its approval in Europe in 2017 and the United States in 2020, there have been no publications describing clinically apparent liver injury due to pitolisant.
Likelihood score: E (unlikely cause of acute liver injury with jaundice).
Mechanism of Injury
The mechanism by which pitolisant might cause liver injury is not known but may be due to a toxic or immunogenic intermediate product of its metabolism. Pitolisant is metabolized in the liver largely by CYP 2D6 and 3A4 and is susceptible to drug-drug interactions with inhibitors or inducers of these enzymes.
Drug Class: CNS Stimulants
Other Drugs for Narcolepsy: Amphetamines, Modafinil, Armodafinil, Methylphenidate, Oxybate, Solriamfetol
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pitolisant – Wakix®
DRUG CLASS
CNS Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
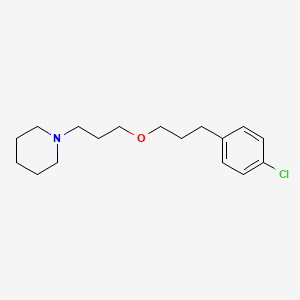
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pitolisant | 362665-56-3 | C17-H26-Cl-N-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 18 August 2021
Abbreviations: CPAP, continuous positive airway pressure; H3, Histamine-3.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of H3 receptor antagonists/inverse agonists).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/211150Orig1s000MedR.pdf. (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA multidisciplinary scientific review of the pitolisant application for safety and efficacy which mentions that rates of ALT elevations during pitolisant therapy were similar to those with placebo and that there was “no pattern of change suggestive of a drug treatment effect”). - Dauvilliers Y, Bassetti C, Lammers GJ, Arnulf I, Mayer G, Rodenbeck A, Lehert P, et al. HARMONY I study group. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12:1068–75. [PubMed: 24107292](Among 95 adults with narcolepsy and excessive daytime sleepiness in an 8 week controlled trial, decreases in excessive sleepiness scores [Epworth Sleepiness Scale] were greater in patients receiving pitolisant [-5.8] than placebo [-3.4] but similar and not greater than with modafinil [-6.8], while adverse events attributed to pitolisant included headache and abdominal discomfort; no mention of ALT elevations or hepatotoxicity).
- Syed YY. Pitolisant: first global approval. Drugs. 2016;76:1313–1318. [PubMed: 27438291](Review of the mechanism of action, development, pharmacology, clinical efficacy and safety of pitolisant shortly after its approval as therapy of narcolepsy by the European Union, mentions adverse events of headache, abdominal pain, increase in appetite, weight gain, insomnia and anxiety; no mention of ALT elevations or hepatotoxicity).
- Szakacs Z, Dauvilliers Y, Mikhaylov V, Poverennova I, Krylov S, Jankovic S, Sonka K, et al. HARMONY-CTP study group. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:200–207. [PubMed: 28129985](Among 106 patients with narcolepsy with cataplexy and excessive daytime sleepiness enrolled in a placebo controlled trial, pitolisant [5 to 20 mg daily] led to a greater decrease in cataplexy episodes per week [9.2 to 2.3: -75%] than did placebo [7.3 to 4.5: -38%] and the adverse event rate was the same in both groups; no mention of ALT elevations or hepatotoxicity).
- Kollb-Sielecka M, Demolis P, Emmerich J, Markey G, Salmonson T, Haas M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017;33:125–129. [PubMed: 28449891](Summary of clinical efficacy and safety of pitolisant in narcolepsy by the European Medicines Agency mentions that adverse events are largely neuropsychiatric such as headache, insomnia, anxiety, irritability, dizziness and depression; no mention of ALT elevations or hepatotoxicity).
- Dauvilliers Y, Arnulf I, Szakacs Z, Leu-Semenescu S, Lecomte I, Scart-Gres C, Lecomte JM, et al. HARMONY III study group. Long-term use of pitolisant to treat patients with narcolepsy: Harmony III Study. Sleep. 2019;42:zsz174. [PMC free article: PMC6802569] [PubMed: 31529094](Among 102 adults with narcolepsy and excessive daytime sleepiness treated with pitolisant [titrated up to as high as 40 mg daily] for one year, sleepiness and episodes of catalepsy decreased and adverse events were mostly mild-to-moderate with headaches [12%], insomnia [9%], weight gain [8%], anxiety [7%], depression [5%], nausea [5%] while “no safety issues were identified regarding….blood chemistry…”).
- Dauvilliers Y, Verbraecken J, Partinen M, Hedner J, Saaresranta T, Georgiev O, Tiholov R, et al. HAROSA II Study Group collaborators. Pitolisant for daytime sleepiness in patients with obstructive sleep apnea who refuse continuous positive airway pressure treatment. A Randomized Trial. Am J Respir Crit Care Med. 2020;201:1135–1145. [PMC free article: PMC7193861] [PubMed: 31917607](Among 267 patients with obstructive sleep apnea not on CPAP therapy who had excessive daytime sleepiness and were treated with pitolisant or placebo for 12 weeks, improvement in Epworth Sleepiness Scale scores were greater with pitolisant [-6.3 vs -3.6 points], while adverse event rates were similar and there were no changes in “blood chemistry” results).
- Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34:9–27. [PMC free article: PMC6982634] [PubMed: 31953791](Review of the mechanism of action, pharmacology, drug-drug interactions, clinical efficacy and safety of newly approved medications for narcolepsy including pitolisant and solriamfetol: no mention of ALT elevations or hepatotoxicity).
- Pépin JL, Georgiev O, Tiholov R, Attali V, Verbraecken J, Buyse B, Partinen M. Fet al.; HAROSA I Study Group. Pitolisant for residual excessive daytime sleepiness in OSA patients adhering to CPAP: a randomized trial. Chest. 2021;159:1598–1609. [PubMed: 33121980](Among 244 patients with obstructive sleep apnea receiving CPAP therapy who had excessive daytime sleepiness and were treated with pitolisant or placebo for 12 weeks, improvements in Epworth Sleepiness Scale were greater with pitolisant than placebo [-6.0 vs -2.6] as were adverse events of headache [15% vs 1.5%] and insomnia [9.3% vs 3.3%]; but “no major changes were found in …laboratory test results during the study”).
- Pitolisant (Wakix) for narcolepsy. Med Lett Drugs Ther. 2021;63(1617):19–21. [PubMed: 33647004](Concise review of the mechanism of action, and relative efficacy, safety and costs of pitolisant in relation to other medications for narcolepsy shortly after its approval for use in the US, mentions side effects of headache, insomnia, nausea and prolongation of the QTc interval).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Pitolisant for treating patients with narcolepsy.[Expert Rev Clin Pharmacol. 2020]Pitolisant for treating patients with narcolepsy.Li S, Yang J. Expert Rev Clin Pharmacol. 2020 Feb; 13(2):79-84. Epub 2020 Jan 23.
- Clinical Impact of Pitolisant on Excessive Daytime Sleepiness and Cataplexy in Adults With Narcolepsy: An Analysis of Randomized Placebo-Controlled Trials.[CNS Drugs. 2022]Clinical Impact of Pitolisant on Excessive Daytime Sleepiness and Cataplexy in Adults With Narcolepsy: An Analysis of Randomized Placebo-Controlled Trials.Meskill GJ, Davis CW, Zarycranski D, Doliba M, Schwartz JC, Dayno JM. CNS Drugs. 2022 Jan; 36(1):61-69. Epub 2021 Dec 21.
- Pitolisant, an inverse agonist of the histamine H3 receptor: an alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness.[Clin Neuropharmacol. 2012]Pitolisant, an inverse agonist of the histamine H3 receptor: an alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness.Inocente C, Arnulf I, Bastuji H, Thibault-Stoll A, Raoux A, Reimão R, Lin JS, Franco P. Clin Neuropharmacol. 2012 Mar-Apr; 35(2):55-60.
- Review pitolisant, a novel histamine-3 receptor competitive antagonist, and inverse agonist, in the treatment of excessive daytime sleepiness in adult patients with narcolepsy.[Health Psychol Res. 2022]Review pitolisant, a novel histamine-3 receptor competitive antagonist, and inverse agonist, in the treatment of excessive daytime sleepiness in adult patients with narcolepsy.Sarfraz N, Okuampa D, Hansen H, Alvarez M, Cornett EM, Kakazu J, Kaye AM, Kaye AD. Health Psychol Res. 2022; 10(3):34222. Epub 2022 May 30.
- Review Pitolisant to Treat Excessive Daytime Sleepiness and Cataplexy in Adults with Narcolepsy: Rationale and Clinical Utility.[Nat Sci Sleep. 2020]Review Pitolisant to Treat Excessive Daytime Sleepiness and Cataplexy in Adults with Narcolepsy: Rationale and Clinical Utility.Guevarra JT, Hiensch R, Varga AW, Rapoport DM. Nat Sci Sleep. 2020; 12:709-719. Epub 2020 Oct 12.
- Pitolisant - LiverToxPitolisant - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...