NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Perindopril is an angiotensin-converting enzyme (ACE) inhibitor used in the therapy of hypertension and stable coronary artery disease. Perindopril is associated with a low rate of transient serum aminotransferase elevations and has been linked to rare instances of acute liver injury.
Background
Perindopril (per in' doe pril) is an ACE inhibitor which is approved for use alone and in combination with other agents in the therapy of hypertension. Like other ACE inhibitors, perindopril inhibits the conversion of angiotensin I, a relatively inactive molecule, to angiotensin II which is the major mediator of vasoconstriction and volume expansion induced by the renin-angiotensin system. Other host enzymes besides that which converts angiotensin I to II may be inhibited as well, which may account for some of the side effects of the ACE inhibitors. Perindopril was approved for use in the United States in 1993, and current indications are for therapy of hypertension and to reduce the risk of cardiovascular mortality in patients with stable coronary artery disease. Perindopril is available in 2, 4 and 8 mg tablets in generic forms and under the trade name Aceon. The typical daily dose in adults is 4 to 8 mg in one or two divided doses, which is administered long term. Common side effects include dizziness, fatigue, headache, cough, gastrointestinal upset and skin rash.
Hepatotoxicity
Perindopril, like other ACE inhibitors, has been associated with a low rate of serum aminotransferase elevations (<2%) that, in controlled trials, was similar to the rate with placebo therapy. These elevations were transient and rarely required dose modification. Instances of jaundice and hepatic injury are listed as potential side effects of perindopril in the product label; however, clinically apparent cases of acute liver injury due to perindopril have yet to be reported in the published literature. Other ACE inhibitors have been associated with rare instances of clinically apparent liver injury, which typically arises 2 to 12 weeks after starting therapy and is associated with a cholestatic pattern of injury which can be severe and prolonged. Immunoallergic manifestations (rash, fever, eosinophilia) are infrequent and most patients do not develop autoantibodies. In addition, rare instances of severe acute hepatocellular injury, sometimes arising 1 to 4 years after starting therapy, have been linked to selected ACE inhibitors, but not specifically to perindopril. Nevertheless, the product label mentions the possibiltiy of drug induced liver injury.
Likelihood score: E* (unproved but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the minor serum aminotransferase elevations associated ACE inhibitors including perindopril is not known. Perindopril is hydrolyzed in the liver to the active metabolite perindoprilat, but undergoes minimal further hepatic metabolism. Idiosyncratic liver injury due to the ACE inhibitors is likely due to a reaction to a minor metabolite.
Outcome and Management
There have been too few instances of perindopril associated liver injury described to provide an overall description of its course and outcome. Most instances of acute liver injury reported with ACE inhibitors have been self limited, but there have been rare reports of acute liver failure due to captopril, enalapril, lisinopril and benazepril and several reports of cholestatic hepatitis due to ACE inhibitors leading to prolonged jaundice and vanishing bile duct syndrome. Patients with severe perindopril induced acute liver injury should avoid use of other ACE inhibitors, although cross sensitivity to liver injury among the members of this class of agents has not always been shown.
References to the safety and potential hepatotoxicity of perindopril are given in the Overview section on the Angiotensin-Converting Enzyme (ACE) Inhibitors.
Drug Class: Antihypertensive Agents, Angiotensin-Converting Enzyme Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Perindopril – Generic, Aceon®
DRUG CLASS
Angiotensin-Converting Enzyme Inhibitors
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Perindopril | 82834-16-0 | C19-H32-N2-O5 |
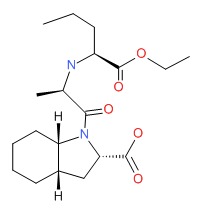 |
- PubChem SubstanceRelated PubChem Substances
- The rationale and design of the PERindopril GENEtic association study (PERGENE): a pharmacogenetic analysis of angiotensin-converting enzyme inhibitor therapy in patients with stable coronary artery disease.[Cardiovasc Drugs Ther. 2009]The rationale and design of the PERindopril GENEtic association study (PERGENE): a pharmacogenetic analysis of angiotensin-converting enzyme inhibitor therapy in patients with stable coronary artery disease.Brugts JJ, de Maat MP, Boersma E, Witteman JC, van Duijn C, Uitterlinden AG, Bertrand M, Remme W, Fox K, Ferrari R, et al. Cardiovasc Drugs Ther. 2009 Apr; 23(2):171-81. Epub 2008 Dec 10.
- Individualized Angiotensin-Converting Enzyme (ACE)-Inhibitor Therapy in Stable Coronary Artery Disease Based on Clinical and Pharmacogenetic Determinants: The PERindopril GENEtic (PERGENE) Risk Model.[J Am Heart Assoc. 2016]Individualized Angiotensin-Converting Enzyme (ACE)-Inhibitor Therapy in Stable Coronary Artery Disease Based on Clinical and Pharmacogenetic Determinants: The PERindopril GENEtic (PERGENE) Risk Model.Oemrawsingh RM, Akkerhuis KM, Van Vark LC, Redekop WK, Rudez G, Remme WJ, Bertrand ME, Fox KM, Ferrari R, Danser AH, et al. J Am Heart Assoc. 2016 Mar 28; 5(3):e002688. Epub 2016 Mar 28.
- The cardioprotective effects of the angiotensin-converting enzyme inhibitor perindopril in patients with stable coronary artery disease are not modified by mild to moderate renal insufficiency: insights from the EUROPA trial.[J Am Coll Cardiol. 2007]The cardioprotective effects of the angiotensin-converting enzyme inhibitor perindopril in patients with stable coronary artery disease are not modified by mild to moderate renal insufficiency: insights from the EUROPA trial.Brugts JJ, Boersma E, Chonchol M, Deckers JW, Bertrand M, Remme WJ, Ferrari R, Fox K, Simoons ML, EUROPA Investigators. J Am Coll Cardiol. 2007 Nov 27; 50(22):2148-55. Epub 2007 Nov 13.
- Review Angiotensin-converting enzyme inhibition by perindopril in the treatment of cardiovascular disease.[Expert Rev Cardiovasc Ther. 2009]Review Angiotensin-converting enzyme inhibition by perindopril in the treatment of cardiovascular disease.Brugts JJ, Ferrari R, Simoons ML. Expert Rev Cardiovasc Ther. 2009 Apr; 7(4):345-60.
- Review Tailored therapy of ACE inhibitors in stable coronary artery disease: pharmacogenetic profiling of treatment benefit.[Pharmacogenomics. 2010]Review Tailored therapy of ACE inhibitors in stable coronary artery disease: pharmacogenetic profiling of treatment benefit.Brugts JJ, Boersma E, Simoons ML. Pharmacogenomics. 2010 Aug; 11(8):1115-26.
- Perindopril - LiverToxPerindopril - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...