NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pentoxifylline is a xanthine derivative that decreases the viscosity of blood and is used to treat symptoms of intermittent claudication due to peripheral vascular disease. Pentoxifylline has not been associated with serum enzyme elevations during therapy, but in several isolated case reports has been linked to clinically apparent liver injury.
Background
Pentoxifylline (pen" tox if' i lin) is a small molecular weight methyl derivative of xanthine that appears to act by improving red blood cell deformability, thus reducing blood viscosity as well as decreasing platelet aggregation and thrombus formation. Several prospective controlled trials have shown that pentoxifylline decreases symptoms of intermittent claudication, although its overall effects appear to be modest. Pentoxifylline is a xanthine and also acts as a nonselective inhibitor of phosphodiesterases, which causes an increase in intracellular cyclic AMP and decreased synthesis of tumor necrosis factor alpha and leukotrienes. These antiinflammatory effects have led to its evaluation in several inflammatory liver diseases such as acute and chronic alcoholic hepatitis, nonalcoholic steatohepatitis (NASH), and autoimmune liver diseases. Pentoxifylline was approved in the United States in 1984 after it had been used extensively in other countries for more than a decade. Current indications are limited to symptomatic therapy of intermittent claudication. Pentoxifylline is available by prescription in extended release tablets of 400 mg in several generic forms and under the brand names Trental and Pentoxil. The recommended dose is 400 mg three times daily with meals. Side effects are not uncommon and can include flushing, abdominal discomfort, diarrhea, dyspepsia, nausea, headache, dizziness and blurred vision. Patients with intolerance to xanthines such as caffeine or theophylline may have similar symptoms with pentoxifylline.
Hepatotoxicity
Chronic therapy with pentoxifylline has not been associated with elevations in serum enzyme levels, although the rigor with which liver test abnormalities were sought in patients taking the drug was not always clear. Despite its use for more than 3 decades, pentoxifylline has been linked to only rare and not completely convincing cases of clinically apparent liver injury. Nevertheless, adverse effects of hepatitis, jaundice, cholestasis and increased liver enzymes are listed in product labels for pentoxifylline. In reported cases, the time to onset was 3 to 4 weeks and the pattern of liver enzyme elevations was distinctly cholestatic (Case 1). Autoimmune and immunoallergic features were not present. The injury was self-limited and there have been no reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome associated with pentoxifylline therapy.
In addition, pentoxifylline has been evaluated as the therapy of several liver diseases including acute alcoholic hepatitis and cirrhosis, nonalcoholic fatty liver disease and autoimmune liver conditions with varying results. In several small controlled trials in severe acute alcoholic hepatitis, pentoxifylline therapy was associated with a significant decrease in short term mortality and in the frequency of the hepatorenal syndrome. However, in large well controlled trials in alcoholic fatty liver, pentoxifylline with or without corticosteroids was found to have no effect on either short- or long-term mortality and minimal or no effect on rates of renal failure. Pentoxifylline has also been reported to improve serum aminotransferase levels and hepatic histology in adult patients with nonalcoholic steatohepatitis (NASH), but these findings have yet to be tested in larger randomized controlled trials. All studies, however, found pentoxifylline well tolerated in patients with liver disease and without evidence of hepatotoxicity.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which pentoxifylline might cause liver injury is not known. It is metabolized extensively by red blood cells and in the liver, but it does not affect the levels of cytochrome P450 activity.
Outcome and Management
While pentoxifylline has been reported to cause clinically apparent liver injury, most cases have been mild and self-limited. Several have occurred in patients with a history of intolerance to caffeine and xanthine derivatives. Thus, patients with xanthine hypersensitivity should avoid use of pentoxifylline.
Drug Class: Cardiovascular Drugs, Intermittent Claudication Agents; Xanthine Derivatives
Other Drugs in the Subclass, Intermittent Claudication Agents: Cilostazol
CASE REPORT
Case 1. Self-limited cholestatic hepatitis due to pentoxifylline.(1)
An 81 year old woman developed fatigue, nausea and dark urine followed by jaundice 3 weeks after starting pentoxifylline (1200 mg daily) for suspected peripheral vascular disease. She denied a history of liver disease, alcohol abuse and risk factors for viral hepatitis. Her other medical problems included asthma for which she had been using oral theophylline (350 mg daily) and a salbutamol inhaler for many years. She was taking no other medications or herbal products and had no history of drug allergy. On presentation, she was jaundiced but had no organomegaly or signs of chronic liver disease. Laboratory tests showed a total bilirubin of 20.1 mg/dL, ALT 577 U/L, AST 293 U/L, GGT 1361 U/L and alkaline phosphatase 1387 U/L (Table). Serum albumin was low (2.4 g/dL), but the INR was normal. Tests for hepatitis A, B and C and for EBV and CMV were negative. Autoantibodies were not present and immunoglobulin levels were normal. Abdominal ultrasound and CT scans were normal without evidence of biliary obstruction, which was verified by endoscopic retrograde cholangiography. A liver biopsy showed mild portal inflammation and centrilobular (zone 3) cholestasis. Pentoxifylline was stopped and jaundice began to resolve. Eight weeks later she was asymptomatic and all liver tests were normal.
Key Points
| Medication: | Pentoxifylline |
|---|---|
| Pattern: | Mixed (R=2.9) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 3 weeks |
| Recovery: | Complete recovery 2 months after stopping |
| Other medications: | Theophylline, salbutamol inhalant |
Laboratory Values
Comment
This was a reasonably convincing case of drug induced, cholestatic hepatitis arising 3 to 4 weeks after starting pentoxifylline. No other cause of jaundice was found despite extensive testing including imaging of the biliary tree by ERCP. While the patient was initially deeply jaundiced, the course was benign and all tests returned to normal within 2 months of stopping pentoxifylline. Interestingly, the patient had asthma and was also taking another xanthine derivative (theophylline) in stable daily doses. Cross sensitivity to other side effects of xanthine derivatives can occur during pentoxifylline therapy, and its combination with full doses of theophylline may have led to excessive xanthine levels and organ toxicity. Cases of hepatitis due to pentoxifylline have been reported to the sponsor, but few have been published and pentoxifylline is not listed in the many large, case series on drug induced liver injury. Thus, hepatotoxicity from pentoxifylline probably occurs, but must be quite rare.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pentoxifylline – Generic, Pentoxil®, Trental®
DRUG CLASS
Intermittent Claudication Agents
Product labeling at DailyMed, National Library of Medicine, NIH
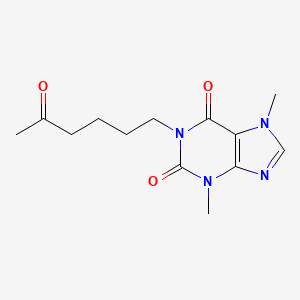
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pentoxifylline | 6493-05-6 | C13-H18-N4-O3 |
|
CITED REFERENCE
- 1.
- Sáez-Royuela F, López-Vázquez A, López-Morante A, Díez-Sánchez V, Martín-Lorente JL. Pentoxifylline-induced acute hepatitis. J Hepatol. 1995;23:482–4. [PubMed: 8655971]
ANNOTATED BIBLIOGRAPHY
References updated: 24 June 2020
- Zimmerman HJ. Pentoxifylline. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 654.(Expert review of hepatotoxicity published in 1999 mentions a single case report of liver injury attributed pentoxifylline [Saez-Royuela 1995] and that several cases have been reported to the FDA).
- Eschenhagen T. Treatment of ischemic heart disease. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 489-506.(Textbook of pharmacology and therapeutics mentions that pentoxifylline is called a rheologic modifier for its effects on increasing the deformability of red blood cells, but that its effects on lower extremity claudication are modest).
- Di Perri T, Guerrini M. Placebo controlled double blind study with pentoxifylline of walking performance in patients with intermittent claudication. Angiology. 1983;34:40–5. [PubMed: 6824189](Among 24 patients with intermittent claudication treated for 30 days, walking performance improved with pentoxifylline but not placebo, and "no adverse reactions were recorded during the trial").
- Reich T, Cutler BC, Lee BY, Porter JM, Reichle FA, Scogin JT, Strandness DE. Pentoxifylline in the treatment of intermittent claudication of the lower limbs. Angiology. 1984;35:389–95. [PubMed: 6380347](Among 128 patients with intermittent claudication treated with pentoxifylline or placebo for 24 weeks, walking ability improved significantly in the pentoxifylline treated patients and "clinical chemistry findings were not affected").
- Roekaerts F, Deleers L. Trental 400 in the treatment of intermittent claudication: results of long-term, placebo-controlled administration. Angiology. 1984;35:396–406. [PubMed: 6380348](Among 36 patients with intermittent claudication treated for 6 months, walking performance improved with pentoxifylline but not placebo, and side effects were minor and there were no changes in "laboratory findings").
- Aviado DM, Dettelbach HR. Pharmacology of pentoxifylline: a hemorheologic agent for the treatment of intermittent claudication. Angiology. 1984;35:407–17. [PubMed: 6380349](Review of the structure, mechanism of action, pharmacology and efficacy of pentoxifylline; no discussion of adverse events, ALT elevations or hepatotoxicity).
- Strano A, Davi G, Avellone G, Novo S, Pinto A. Double-blind, crossover study of the clinical efficacy and the hemorheological effects of pentoxifylline in patients with occlusive arterial disease of the lower limbs. Angiology. 1984;35:459–66. [PubMed: 6380350](Among 18 patients with intermittent claudication treated for 3 months, walking distance increased by 46% with pentoxifylline vs 4% with placebo, which paralleled changes in blood viscosity; no mention of adverse events).
- Gallus AS, Gleadow F, Dupont P, Walsh J, Morley AA, Wenzel A, Alderman M, Chivers D. Intermittent claudication: a double-blind crossover trial of pentoxifylline. Aust N Z J Med. 1985;15:402–9. [PubMed: 3866536](Among 38 patients with intermittent claudication treated with pentoxifylline or placebo for 8 weeks, there were no differences in changes in treadmill walking or red blood cell filterability between the two groups).
- Reilly DT, Quinton DN, Barrie WW. A controlled trial of pentoxifylline (Trental 400) in intermittent claudication: clinical, haemostatic and rheological effects. N Z Med J. 1987;100:445–7. [PubMed: 3330188](Among 30 patients with intermittent claudication treated with pentoxifylline or placebo, both groups had a similar decrease in symptoms and improvement in time to claudication; red blood cell filterability did not correlate with claudication distance).
- Cameron HA, Waller PC, Ramsay LE. Drug treatment of intermittent claudication: a critical analysis of the methods and findings of published clinical trials, 1965-1985. Br J Clin Pharmacol. 1988;26:569–76. [PMC free article: PMC1386634] [PubMed: 3061424](Analysis of 75 trials of 33 drugs used to treat intermittent claudication in the English literature [1965-1985] found design problems in 76% of trials and concluded that available information does not support the efficacy of any agent in improving exercise performance in intermittent claudication).
- Sáez-Royuela F, López-Vázquez A, López-Morante A, Díez-Sánchez V, Martín-Lorente JL. Pentoxifylline-induced acute hepatitis. J Hepatol. 1995;23:482–4. [PubMed: 8655971](81 year old woman developed jaundice 3 weeks after starting pentoxifylline and while receiving theophylline for asthma [bilirubin 20.1 mg/dL, ALT 577 U/L, Alk P 1387 U/L], resolving within 8 weeks of stopping: Case 1).
- Dawson DL, Cutler BS, Hiatt WR, Hobson RW 2nd, Martin JD, Bortey EB, Forbes WP, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523–30. [PubMed: 11063952](Among 698 patients with intermittent claudication treated with cilostazol [100 mg twice daily], pentoxifylline [400 mg thrice daily] or placebo, maximal walking distance improved more with cilostazol [54%] than pentoxifylline [30%], which was no better than placebo [34%]; adverse events were similar in all 3 groups; no mention of ALT levels or hepatotoxicity).
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. [PubMed: 11113085](Among 101 patients with severe alcoholic hepatitis treated with pentoxifylline or placebo for 4 weeks, hospital mortality was 46% in placebo recipients vs 24.5% in pentoxifylline treated subjects, a difference that was sustained during 6 month follow up; there were no drug related serious adverse events or evidence of hepatotoxicity).
- Morgan TR, McClain CJ. Pentoxifylline and alcoholic hepatitis. Gastroenterology. 2000;119:1787–91. [PubMed: 11113103](Editorial in response to Akriviadis [1995] commenting on the dramatic findings of reduced mortality and lower rate of hepatorenal syndrome in those receiving pentoxifylline, and concluding that further evaluation of pentoxifylline in alcoholic liver disease was warranted).
- Bharucha AE, Jorgensen R, Lichtman SN, LaRusso NF, Lindor KD. A pilot study of pentoxifylline for the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2338–42. [PubMed: 11007239](Among 20 patients with sclerosing cholangitis treated with pentoxifylline for up to one year, symptoms and abnormal liver tests did not improve, 2 patients stopped therapy because of symptoms of nausea, but there was no evidence of hepatotoxicity).
- De Sanctis MT, Cesarone MR, Belcaro G, Nicolaides AN, Griffin M, Incandela L, Bucci M, et al. Treatment of intermittent claudication with pentoxifylline: a 12-month, randomized trial--walking distance and microcirculation. Angiology. 2002 Jan-Feb;53 Suppl 1:S7–12. [PubMed: 11865838](Among 101 patients with intermittent claudication treated with pentoxifylline or placebo for 12 months, improvements in walking distance were greater with pentoxifylline than placebo [404% vs 280%] and "no serious drug-related side effects were observed").
- Cesarone MR, Belcaro G, Nicolaides AN, Griffin M, De Sanctis MT, Incandela L, Geroulakos G, et al. Treatment of severe intermittent claudication with pentoxifylline: a 40-week, controlled, randomized trial. Angiology. 2002;53 Suppl 1:S1–5. [PubMed: 11865828](Among 178 patients with intermittent claudication treated with pentoxifylline or placebo for 40 weeks, total walking distance improved more pentoxifylline [330% vs 183%] and "no serious drug-related side effects [which would require discontinuation] were observed").
- Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. Am J Cardiovasc Drugs. 2003;3(2):117–38. [PubMed: 14727939](Review of the mechanism of action, efficacy and safety of cilostazol mentions that side effects are common, but generally well tolerated and cilostazol has significant drug-drug interactions with CYP 3A4 and 2C19 inducers; no mention of ALT elevations or hepatotoxicity).
- Drugs for intermittent claudication. Med Lett Drugs Ther 2004; 46 1176): 13-5. [PubMed: 14973403](Concise review of the medical management of intermittent claudication mentions that pentoxifylline is approved for symptomatic therapy of this condition, but that its efficacy is controversial; no mention of ALT elevations or hepatotoxicity).
- Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–8. [PubMed: 15571584](20 patients with NASH were treated with pentoxifylline or placebo for up to one year, but almost half did not complete therapy because of side effects; ALT and AST levels decreased in patients who continued therapy, but no histology was presented; no mention of hepatotoxicity).
- Lee YM, Sutedja DS, Wai CT, Dan YY, Aung MO, Zhou L, Cheng CL, et al. A randomized controlled pilot study of pentoxifylline in patients with non-alcoholic steatohepatitis (NASH). Hepatol Int. 2008;2:196–201. [PMC free article: PMC2716847] [PubMed: 19669304](Among 20 patients with NASH who were treated with pentoxifylline [n=11] or placebo for 12 weeks, improvements in weight, ALT and cytokine levels occurred in both groups).
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613–9. [PMC free article: PMC2669113] [PubMed: 19340904](Among 68 patients with severe acute alcoholic hepatitis treated with pentoxifylline [1200 mg daily] or prednisone [40 mg daily] for 28 days with extension to 3 months, mortality was less with pentoxifylline [15%] than prednisone [35%], the major difference being in fatal hepatorenal syndrome [0% vs 18%]).
- Stevens JW, Simpson E, Harnan S, Squires H, Meng Y, Thomas S, Michaels J, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg. 2012;99:1630–8. [PubMed: 23034699](Systematic review of the literature on drugs for intermittent claudication concluded that pentoxifylline was less effective than cilostazol, that adverse events were "generally minor" and that serious adverse events were not increased; no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to pentoxifylline).
- Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, Saliba F, et al. Pentocir Group. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755–62. [PubMed: 20102716](Among 335 patients with alcoholic cirrhosis treated with pentoxifylline or placebo for 6 months, mortality was similar in both groups [30% vs 31%], but liver related complications were less with pentoxifylline [21% vs 37%]; no mention of worsening of ALT levels or hepatotoxicity).
- Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. [PMC free article: PMC3205292] [PubMed: 21748765](Among 55 patients with NASH treated with pentoxifylline or placebo for 1 year, liver histology improved in 38% vs 14%, and rates of ALT improvement were also higher with pentoxifylline; no mention of hepatotoxicity).
- Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, Rinella ME. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–86. [PubMed: 21677329](Among 30 patients with NASH treated with pentoxifylline or placebo for 12 months, ALT levels and histology scores improved similarly in both groups; there were no severe adverse reactions or worsening of ALT levels).
- Sharma BC, Kumar A, Garg V, Reddy RS, Sakhuja P, Sarin SK. A randomized controlled trial comparing efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non-alcoholic steatohepatitis. J Clin Exp Hepatol. 2012;2:333–7. [PMC free article: PMC3940593] [PubMed: 25755455](Among 60 adults with NASH treated with pentoxifylline or pioglitazone for 12 months, ALT levels and liver histology improved in both groups, and there were no differences in adverse reactions and no increases in ALT levels or clinically apparent liver injury).
- Sidhu SS, Goyal O, Singla M, Bhatia KL, Chhina RS, Sood A. Pentoxifylline in severe alcoholic hepatitis: a prospective, randomised trial. J Assoc Physicians India. 2012;60:20–2. [PubMed: 23029716](Among 50 patients with severe alcoholic hepatitis treated with pentoxifylline or placebo for 4 weeks, mortality was less with pentoxifylline [20% vs 40%] at 4 weeks; the major difference being in deaths accompanied by renal failure).
- Saunderson RB, Garsia R, Headley AP, McCaughan GW, O'Toole S, Strasser SI. Pentoxifylline-induced drug rash with eosinophilia and systemic symptoms (DRESS) in a patient with caffeine intolerance. J Dermatol Case Rep. 2013;7:77–81. [PMC free article: PMC3797013] [PubMed: 24133560](39 year of man with severe acute alcoholic hepatitis [bilirubin 20 mg/dL, ALT 64 U/L, AST 328 U/L, Alk P 231 U/L, prothrombin time 18 sec] was treated with pentoxifylline, as well as ticarcillin with clavulanate and developed fever, rash, eosinophilia and worsening hepatic function, improving on stopping both; had a history of caffeine intolerance, but also drug rash from amoxicillin/clavulanate).
- Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, Anty R, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033–41. [PubMed: 24026598](Among 270 patients with severe alcoholic hepatitis treated with prednisone with or without pentoxifylline for 28 days, 1 and 6 month survival were the same in both groups, although hepatorenal syndrome was less at 4 weeks with pentoxifylline [3% vs 12%]; no mention of possible hepatotoxicity).
- Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, Sohn JH, et al. Korean Association for the Study of the Liver (KASL)-Alcohol Related Problems Study Group. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. 2014;61:792–8. [PubMed: 24845609](Among 121 patients with severe alcoholic hepatitis treated with pentoxifylline or prednisone for 1 month, 1 and 6 month survival rates were not different; no mention of worsening of ALT levels or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to pentoxifylline).
- Baniasadi N, Salajegheh F, Pardakhty A, Seyedmirzaee SM, Hayatbakhsh MM, Nikpoor AR, Mohammadi M. Effects of pentoxifylline on non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial in Iran. Hepat Mon. 2015;15:e32418. [PMC free article: PMC4719129] [PubMed: 26834792](Among 30 patients with nonalcoholic steatohepatitis treated with pentoxifylline or placebo for 24 weeks, ALT levels improved in both groups to a similar degree; no mention of adverse events or ALT elevations).
- Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, et al. STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–28. [PubMed: 25901427](Among 1053 patients with severe alcoholic hepatitis treated with pentoxifylline or prednisone or both or neither, survival rates at 1, 3 and 12 months were not different in the four groups and there were no differences in rates or types of serious adverse reactions including renal failure [3-5%]).
- Thursz M, Forrest E, Roderick P, Day C, Austin A, O'Grady J, Ryder S, et al. The clinical effectiveness and cost-effectiveness of STeroids Or Pentoxifylline for Alcoholic Hepatitis (STOPAH): a 2 × 2 factorial randomized controlled trial. Health Technol Assess. 2015;19:1–104. [PMC free article: PMC4781103] [PubMed: 26691209](Among 1053 patients with acute alcoholic hepatitis treated with pentoxifylline or prednisone or both or placebo, 90 day mortality was similar with or without pentoxifylline [29.1% vs 29.8%] and with or without prednisone [29.8% vs 29.1%], and serious adverse events were frequent but similar with or without pentoxifylline [41% vs 39%]).
- Cioboată R, Găman A, Traşcă D, Ungureanu A, Docea AO, Tomescu P, Gherghina F, et al. Pharmacological management of non-alcoholic fatty liver disease: atorvastatin versus pentoxifylline. Exp Ther Med. 2017;13:2375–81. [PMC free article: PMC5443168] [PubMed: 28565851](Among 98 patients with nonalcoholic fatty liver disease treated with atorvastatin [if dyslipidemic] or pentoxifylline [if not] for an average of 32 weeks, ALT and steatosis scores improved in both groups; no mention of adverse events).
- Alam S, Nazmul Hasan S, Mustafa G, Alam M, Kamal M, Ahmad N. Effect of pentoxifylline on histological activity and fibrosis of nonalcoholic steatohepatitis patients: a one year randomized control trial. J Transl Int Med. 2017;5:155–63. [PMC free article: PMC5655462] [PubMed: 29085788](Among 35 patients with nonalcoholic steatohepatitis treated with pentoxifylline [400 mg twice daily] or placebo for 1 year, ALT and AST levels improved in both groups and to a similar degree as did steatosis; no mention of hepatic adverse events).
- Stine JG, Wang J, Cornella SL, Behm BW, Henry Z, Shah NL, Caldwell SH, Northup PG. Treatment of type-1 hepatorenal syndrome with pentoxifylline: a randomized placebo controlled clinical trial. Ann Hepatol. 2018;17:300–6. [PMC free article: PMC7485043] [PubMed: 29469046](Among 12 patients with cirrhosis and hepatorenal syndrome treated with pentoxifylline or placebo for up to 10 days, the two groups were similar in rates of resolution of renal dysfunction and overall survival, while pentoxifylline was well tolerated in these very ill patients with no adverse events attributed to its use).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Pharmacology of pentoxifylline, a hemorheologic agent for the treatment of intermittent claudication.[Angiology. 1984]Pharmacology of pentoxifylline, a hemorheologic agent for the treatment of intermittent claudication.Aviado DM, Dettelbach HR. Angiology. 1984 Jul; 35(7):407-17.
- Review Pentoxifylline for intermittent claudication.[Cochrane Database Syst Rev. 2015]Review Pentoxifylline for intermittent claudication.Salhiyyah K, Forster R, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA. Cochrane Database Syst Rev. 2015 Sep 29; 9(9):CD005262. Epub 2015 Sep 29.
- Failure of pentoxifylline or cilostazol to improve blood and plasma viscosity, fibrinogen, and erythrocyte deformability in claudication.[Angiology. 2002]Failure of pentoxifylline or cilostazol to improve blood and plasma viscosity, fibrinogen, and erythrocyte deformability in claudication.Dawson DL, Zheng Q, Worthy SA, Charles B, Bradley DV Jr. Angiology. 2002 Sep-Oct; 53(5):509-20.
- Review Pentoxifylline for intermittent claudication.[Cochrane Database Syst Rev. 2012]Review Pentoxifylline for intermittent claudication.Salhiyyah K, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA. Cochrane Database Syst Rev. 2012 Jan 18; 1:CD005262. Epub 2012 Jan 18.
- Pentoxifylline--a new drug for the treatment of intermittent claudication.[Indian Heart J. 1989]Pentoxifylline--a new drug for the treatment of intermittent claudication.Chopra HK, Chopra KL, Aggarwal KK, Parashar SK. Indian Heart J. 1989 Mar-Apr; 41(2):127-33.
- Pentoxifylline - LiverToxPentoxifylline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...