NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pemetrexed is a parenterally administered folate antagonist and antineoplastic agent, used in the treatment of non-small cell lung cancer and malignant mesothelioma. Pemetrexed therapy has been associated with moderate rates of serum enzyme elevations during therapy, but has not been convincingly linked to instances of acute, clinically apparent liver injury.
Background
Pemetrexed (pem" e trex' ed) is a folic acid analog which acts as an antagonist to the enzymes involved in folate dependent synthetic pathways such as thymidine synthase, dihydrofolate reductase and glycinamide ribonucleotide formyltransferase. Inhibition of these enzymes leads to decrease in intracellular thymidine and purine which interferes with DNA synthesis and leads to apoptotic cell death in rapidly dividing cells. In vitro and in vivo studies have shown that pemetrexed inhibits growth of mesothelioma and non-small cell lung cancer cell lines, and clinical trials in advanced forms of these cancers have shown improvements in overall survival times in pemetrexed treated subjects. Pemetrexed was approved for use in the United States in 2004. Current indications are for locally advanced or metastatic nonsquamous, non-small cell lung cancer (NSCLC) and for malignant pleural mesothelioma, usually in combination with other antineoplastic agents (such as cisplatin) and with folic acid and vitamin B12 supplementation. Pemetrexed is available in single use vials of 100 or 500 mg as a powder for reconstitution under the brand name Alimta. The recommended dose is 500 mg/m2 intravenously on day 1 of each 21 day cycle. Pemetrexed shares common side effects with other folate antagonists such as fatigue, nausea, vomiting, anorexia, diarrhea, alopecia, bone marrow suppression, and rash. Uncommon, but potentially serious adverse events include febrile neutropenia, infections, dehydration, renal failure, arrhythmias and peripheral neuropathy.
Hepatotoxicity
Pemetrexed therapy is associated with a low-to-moderate rate of serum enzyme elevations, but these are generally mild, transient and without accompanying symptoms or jaundice. Serum ALT or AST elevations above 5 times ULN occur in 1% to 6% of patients, but are usually self-limited in course and rarely require dose modification or discontinuation. No instances of clinically apparent acute liver injury attributed to pemetrexed have been reported. In addition, pemetrexed has not been linked to sinusoidal obstruction syndrome or to reactivation of hepatitis B, but it is rarely used in high doses in neoplastic disease or in conditioning regimens for bone marrow transplantation, situations in which other neoplastic agents are commonly associated with these complications.
Likelihood score: E* (unlikely but suspected cause of liver injury).
Mechanism of Injury
The cause of the serum enzyme elevations that may occur during pemetrexed use is probably direct hepatotoxicity from the folate antagonism. Its hepatic metabolism is minimal and most of the administered dose is excreted unchanged in the urine.
Outcome and Management
Liver injury is rare after pemetrexed therapy. Persistent serum enzyme elevations above 5 times ULN should lead to temporary discontinuation of pemetrexed therapy, but should also lead to careful search for other possible causes of liver injury. There have been no instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome linked to pemetrexed therapy.
Drug Class: Antineoplastic Agents, Antifolate Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pemetrexed – Alimta®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pemetrexed | 150399-23-8 | C20-H21-N5-O6.2Na |
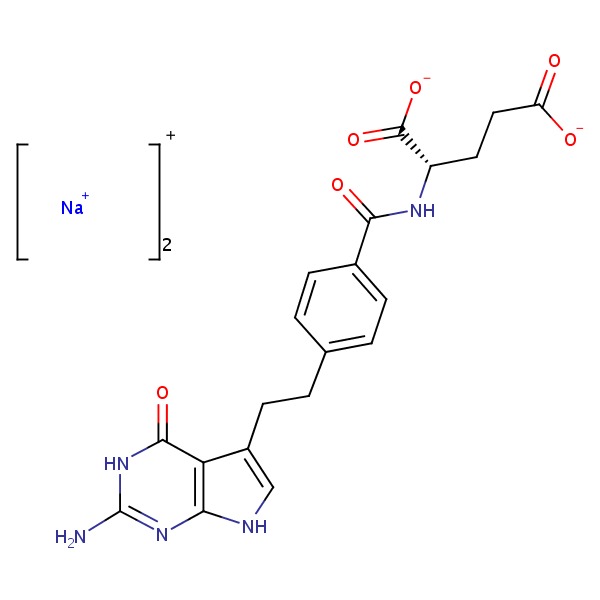 |
| Folate | 6484-89-5 | C19-H18-N7-O6.Na |
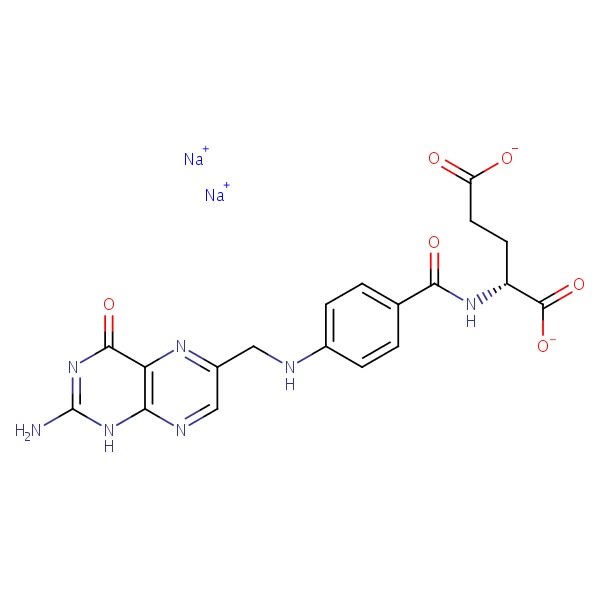 |
ANNOTATED BIBLIOGRAPHY
References updated: 18 April 2016
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; pemetrexed is not discussed).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; pemetrexed is not discussed).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Folic acid analogs. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1690-94.(Textbook of pharmacology and therapeutics).
- Rusthoven JJ, Eisenhauer E, Butts C, Gregg R, Dancey J, Fisher B, Iglesias J. Multitargeted antifolate LY231514 as first-line chemotherapy for patients with advanced non-small-cell lung cancer: A phase II study. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1999; 17: 1194. [PubMed: 10561178](Among 33 patients with NSCLC treated with pemetrexed [500-600 mg/m2 iv every 3 weeks], partial responses occurred in 23%, but there was a high rate of adverse events including fatigue in 80%, rash 79%, anorexia 67%, diarrhea 42% and AST elevations in 84% which were above 5 times ULN in 6%).
- Clarke SJ, Abratt R, Goedhals L, Boyer MJ, Millward MJ, Ackland SP. Phase II trial of pemetrexed disodium (ALIMTA, LY231514) in chemotherapy-naïve patients with advanced non-small-cell lung cancer. Ann Oncol 2002; 13: 737-41. [PubMed: 12075742](Among 55 patients with NSCLC treated with pemetrexed [600 mg/m2 iv every 3 weeks], overall response rate was 15% and side effects were common, rash in 83%, nausea 76%, fatigue 56%, mucositis 44%, diarrhea 37% and ALT or AST elevations in 80% which were above 5 times ULN in 20%, but abnormalities often improved despite continuing therapy and no patient required drug discontinuation for liver related abnormalities).
- Scagliotti GV, Shin DM, Kindler HL, Vasconcelles MJ, Keppler U, Manegold C, Burris H, et al. Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 2003; 21: 1556-61. [PubMed: 12697881](Among 64 patients with malignant pleural mesothelioma treated with pemetrexed [500 mg/m2 iv every 3 weeks], the response rate was 14% and ALT values above 5 times ULN occurred in 3.1%).
- Cohen MH, Johnson JR, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: pemetrexed for injection (Alimta) for the treatment of non-small cell lung cancer. Oncologist 2005; 10: 363-8. [PubMed: 15967829](Summary of clinical evidence in support of FDA approval of pemetrexed for NSCLC; in a trial of 571 patients treated with pemetrexed or docetaxel, mean survival time and response rates were similar, while pemetrexed treated subjects had lower rates of neutropenia [11% vs 45%] and infections [31% vs 33%], but higher rates of ALT elevations [10% vs 2%] including those above 5 times ULN in [3% vs <1%]).
- Hazarika M, White RM Jr, Booth BP, Wang YC, Ham DY, Liang CY, Rahman A, et al. Pemetrexed in malignant pleural mesothelioma. Clin Cancer Res 2005; 11: 982-92. [PubMed: 15709163](Summary of clinical evidence in support of FDA approval of pemetrexed for malignant mesothelioma; in a trial of 448 patients treated with cisplatin alone or in combination with pemetrexed, median survival time increased from 9.3 to 12.1 months with addition of the antifolate; AST elevations occurred in 8.3% on pemetrexed [none above 5 times ULN] and cisplatin and in 8.6% on cisplatin alone).
- Jänne PA, Wozniak AJ, Belani CP, Keohan ML, Ross HJ, Polikoff JA, Mintzer DM, et al.; Pemetrexed expanded access program investigators. Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac Oncol 2006; 1: 506-12. [PubMed: 17409909](Among 187 patients with mesothelioma enrolled in an expanded access program, overall response rate was 32.5% with the combination of cisplatin and pemetrexed vs only 5.5% with pemetrexed alone; adverse reactions included dehydration, nausea and pulmonary embolism; no mention of ALT elevations or hepatotoxicity).
- Llombart-Cussac A, Martin M, Harbeck N, Anghel RM, Eniu AE, Verrill MW, Neven P, et al. A randomized, double-blind, phase II study of two doses of pemetrexed as first-line chemotherapy for advanced breast cancer. Clin Cancer Res 2007; 13: 3652-9. [PubMed: 17575230](Among 92 women with advanced breast cancer treated with pemetrexed [600 or 900/m2 every 3 weeks], response rates were 17% and 16% and side effects included ALT elevations above 5 times ULN in 2.1% and 4.4%).
- Taylor P, Castagneto B, Dark G, Marangolo M, Scagliotti GV, van Klaveren RJ, Labianca R, et al. Single-agent pemetrexed for chemonaïve and pretreated patients with malignant pleural mesothelioma: results of an International Expanded Access Program. J Thorac Oncol 2008; 3: 764-71. [PubMed: 18594323](Among 812 patients with mesothelioma treated with pemetrexed in an open access program, partial responses occurred in 10% to 12% of patients and deaths attributed to adverse events in 1.5%; liver test elevations were not recorded).
- Kuribayashi K, Voss S, Nishiuma S, Arakawa K, Nogi Y, Mikami K, Kudoh S. Safety and effectiveness of pemetrexed in patients with malignant pleural mesothelioma based on all-case drug-registry study. Lung Cancer 2012; 75: 353-9. [PubMed: 21890228](Among 903 Japanese patients with mesothelioma monitored in a registry of pemetrexed treated subjects, the overall response rate was 25%, and 1.5% developed interstitial lung disease; no mention of ALT elevations or clinically apparent liver injury).
- Gridelli C, de Marinis F, Pujol JL, Reck M, Ramlau R, Parente B, Pieters T, et al. Safety, resource use, and quality of life in paramount: a phase III study of maintenance pemetrexed versus placebo after induction pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol 2012; 7: 1713-21. [PubMed: 23059776](Among 539 patients with NSCLC treated with maintenance pemetrexed or placebo, adverse events that were more frequent with pemetrexed included fatigue [16% vs 11%], anorexia [4.3% vs 0.5%], nausea [12% vs 2%] and stomatitis [5% vs 2%]; ALT levels not mentioned).
- Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31: 2895-902. [PubMed: 23835707](Among 939 patients with NSCLC treated with an induction regimen of pemetrexed with cisplatin followed by maintenance pemetrexed vs placebo, median overall survival time was 13.9 vs 11.0 months, while ALT elevations were more frequent with pemetrexed [any elevation in 2.5% vs 0.6%, above 5 times ULN in 0.3% vs 0%]).
- Pujol JL, Paz-Ares L, de Marinis F, Dediu M, Thomas M, Bidoli P, Corral J, et al. Long-term and low-grade safety results of a phase III study (PARAMOUNT): maintenance pemetrexed plus best supportive care versus placebo plus best supportive care immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2014; 15: 418-25. [PubMed: 25104617](Among 539 patients with NSCLC given maintenance treatment with pemetrexed or placebo, ALT elevations occurred in 2.8% vs 0.6% and were above 5 times ULN in 0.3% vs 0%).
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Pralatrexate.[LiverTox: Clinical and Researc...]Review Pralatrexate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Trimetrexate.[LiverTox: Clinical and Researc...]Review Trimetrexate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Commentary: a case for minimizing folate supplementation in clinical regimens with pemetrexed based on the marked sensitivity of the drug to folate availability.[Oncologist. 2007]Commentary: a case for minimizing folate supplementation in clinical regimens with pemetrexed based on the marked sensitivity of the drug to folate availability.Chattopadhyay S, Tamari R, Min SH, Zhao R, Tsai E, Goldman ID. Oncologist. 2007 Jul; 12(7):808-15.
- Safety of Same-Day Vitamin B12 Supplementation in Patients Receiving Pemetrexed for the Treatment of Non-Small-Cell Lung Cancer or Pleural Mesothelioma: A Retrospective Analysis.[Clin Lung Cancer. 2018]Safety of Same-Day Vitamin B12 Supplementation in Patients Receiving Pemetrexed for the Treatment of Non-Small-Cell Lung Cancer or Pleural Mesothelioma: A Retrospective Analysis.Schlei Z, Tan W, Faber MG, Chen H, Meagher A, Dy GK. Clin Lung Cancer. 2018 Nov; 19(6):467-475. Epub 2018 Jun 5.
- Review Eravacycline.[LiverTox: Clinical and Researc...]Review Eravacycline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Pemetrexed - LiverToxPemetrexed - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...