NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Orphenadrine is a centrally acting muscle relaxant that has been in clinical use for more than 50 years and has not been linked to liver injury or clinically apparent drug induced liver disease.
Background
Orphenadrine (or fen' a dreen) is a centrally acting, nonopiate analgesic and muscle relaxant. It is a methyl derivative of diphenhydramine (a commonly used antihistamine), but its mechanism of action in causing analgesia and skeletal muscle relaxation is not well defined. Orphenadrine has anticholinergic activity and may act centrally on pain perception. Orphenadrine is currently used for the treatment of acute, painful musculoskeletal conditions and can be given orally or parenterally. Orphenadrine was approved for use as a muscle relaxant in the United States in 1957 and it is still in wide use. Orphenadrine is available in multiple generic forms as standard and extended release tablets of 100 mg. It is also available under commercial names such as Norgesic, Norflex, Deenar, Banflex, Disipal and X-Otag. The recommended dosage is 100 mg twice daily. Orphenadrine is also available in parenteral formulations under the names of Flexoject and Myolin. The parenteral dose recommendation is 60 mg either intravenously or intramuscularly twice daily. The most common side effects are those typical of anticholinergics including drowsiness, dry mouth, diaphoresis, flushing, confusion and visual disturbances. Orphenadrine also has a potential for abuse and fatal overdoses have been reported.
Hepatotoxicity
Despite its long clinical use, there is no evidence of hepatotoxicity with orphenadrine. Several cases of severe orphenadrine overdose with cardio-respiratory arrest and ischemic hepatic injury have been reported. Conventional doses of orphenadrine appear to be free of hepatic injury.
Likelihood score: E (Unlikely cause of clinically apparent liver injury).
Drug Class: Muscle Relaxants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Orphenadrine – Generic, Norflex®
DRUG CLASS
Autonomic Agents: Muscle Relaxants, Central
Product labeling at DailyMed, National Library of Medicine, NIH
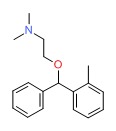
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Orphenadrine | 83-98-7 | C18-H23-N-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 30 January 2017
- Zimmerman HJ. Muscle spasmolytics. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 544-45.(Expert review of hepatotoxicity published in 1999; discusses dantrolene, chlorzoxazone and baclofen, but not orphenadrine).
- Hibbs RE, Zambon AC. Agents acting at the neuromuscular junction and autonomic ganglia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011. p. 255-76.(Textbook of pharmacology and therapeutics).
- Blomquist M, Bonnichsen R, Schubert B. Lethal orphenadrine intoxications. A report of five cases. Z Rechtsmed 1971; 68: 111-4. [PubMed: 5555178](Case reports of fatalities from suicidal overdose of orphenadrine; most died within hours of ingestion and were found dead or died in status epilepticus with few details and no evidence for hepatic injury).
- Bozza-Marrubini M, Frigerio A, Ghezzi R, Parelli L, Restelli L, Selenati A. Two cases of severe orphenadrine poisoning with atypical features. Acta Pharmacol Toxicol (Copenh) 1977; 41 Suppl 2: 137-52. [PubMed: 302558](Eight cases of overdose. Central nervous system toxicity predominates with seizures and cardio-pulmonary arrest; one case had liver test abnormalities with ALT 236 U/L).
- de Mercurio D, Chiarotti M, Giusti GV. Lethal orphenadrine intoxication: report of a case. Z Rechtsmed 1979; 82: 349-53. [PubMed: 433466](Case report of a patient with orphenadrine overdose, found comatose and in cardiac arrest; AST levels were high [1600 U/L]. Autopsy showed evidence of ischemic injury to the liver, most likely due to cardiac arrest rather than direct hepatotoxicity).
- Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage 2004; 28: 140-75. [PubMed: 15276195](Thorough review of the pharmacology, efficacy and side effects of the muscle relaxants).
- Mao YC, Hung DZ, Yang CC, Wang JD. Full recovery from a potentially lethal dose of orphenadrine ingestion using conservative treatment: a case report. Hum Exp Toxicol. 2010; 29: 961-3. [PubMed: 20200196](46 year old woman took overdose [4 g] of orphenadrine and within 4 hours developed stupor, coma and seizures, but ultimately recovered despite rhabdomyolysis; no mention of liver injury).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 [0.7%] were attributed to a muscle relaxant, but none to orphenadrine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Comparison of depressant actions of orphenadrine and diazepam on hypertonic skeletal muscle activity.[Arch Int Pharmacodyn Ther. 1989]Comparison of depressant actions of orphenadrine and diazepam on hypertonic skeletal muscle activity.Williamson HE. Arch Int Pharmacodyn Ther. 1989 Sep-Oct; 301:112-21.
- Review Clinical and pharmacological review of the efficacy of orphenadrine and its combination with paracetamol in painful conditions.[J Int Med Res. 1991]Review Clinical and pharmacological review of the efficacy of orphenadrine and its combination with paracetamol in painful conditions.Hunskaar S, Donnell D. J Int Med Res. 1991 Mar-Apr; 19(2):71-87.
- Results with a centrally acting muscle relaxant in myogenic inflammatory types of trismus.[Quintessence Int (Berl). 1970]Results with a centrally acting muscle relaxant in myogenic inflammatory types of trismus.Nehlmeyer C. Quintessence Int (Berl). 1970 Sep; 1(9):9-12.
- A controlled clinical trial of chlormezanone, orphenadrine, orphenadrine/paracetamol and placebo in the treatment of painful skeletal muscle spasms.[Ann Clin Res. 1975]A controlled clinical trial of chlormezanone, orphenadrine, orphenadrine/paracetamol and placebo in the treatment of painful skeletal muscle spasms.Valtonen EJ. Ann Clin Res. 1975 Apr; 7(2):85-8.
- Review Baclofen.[LiverTox: Clinical and Researc...]Review Baclofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Orphenadrine - LiverToxOrphenadrine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...