NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nitisinone is an inhibitor of the tyrosine catabolism that is used to treat hereditary tyrosinemia, type 1, in which accumulation of intermediates of tyrosine metabolism causes severe and progressive hepatic and renal injury. Nitisinone has been associated with mild, transient serum aminotransferase elevations, but has not been linked to instances of clinically apparent acute liver injury or jaundice.
Background
Nitisinone (nye tis’ i none) is a small molecule inhibitor of 4-hydroxyphenylpyruvate the second enzymatic step in tyrosine metabolism (see Figure 1). Developed initially as an herbicide, it was later found to be beneficial in hereditary type 1 tyrosinemia in which the absence of fumarylacetoacetate hydrolase (FAH), the fifth step in tyrosine catabolism, causes buildup of toxic intermediates that cause early onset liver failure and severe renal tubular dysfunction. Treatment of infants and children with nitisinone combined with a low protein diet led to reversal of liver failure and correction of renal tubular dysfunction, allowing for normal growth and development. Children treated before the onset of liver failure avoided the need for liver transplantation and did not develop hepatocellular carcinoma, common outcomes before the availability of this agent. Nitisinone was approved as an orphan drug for use in tyrosinemia, type 1, in the United States in 2002. Nitisinone is available in tablets of 2, 5, 10 and 20 mg and as an oral suspension of 4 mg/mL under the brand name Orfadin. The recommended dose is 0.5 mg/kg orally given twice daily, but monitoring with drug and tyrosine levels is usually recommended and can lead to dose modification. Side effects are infrequent, but serious complications include corneal irritation, opacities and ulcers, skin rash, developmental delay and intellectual disability, leukopenia and thrombocytopenia. Most of these serious side effects of nitisinone are thought to be due to the elevation in plasma tyrosine levels which typically increase ten-fold during treatment. These increases can be partially prevented by limiting tyrosine and phenylalanine intake by a low or limited protein diet, a necessary component in the therapy of this disease.
Figure 1. Metabolic Disorders of Tyrosine and Nitisinone Action.
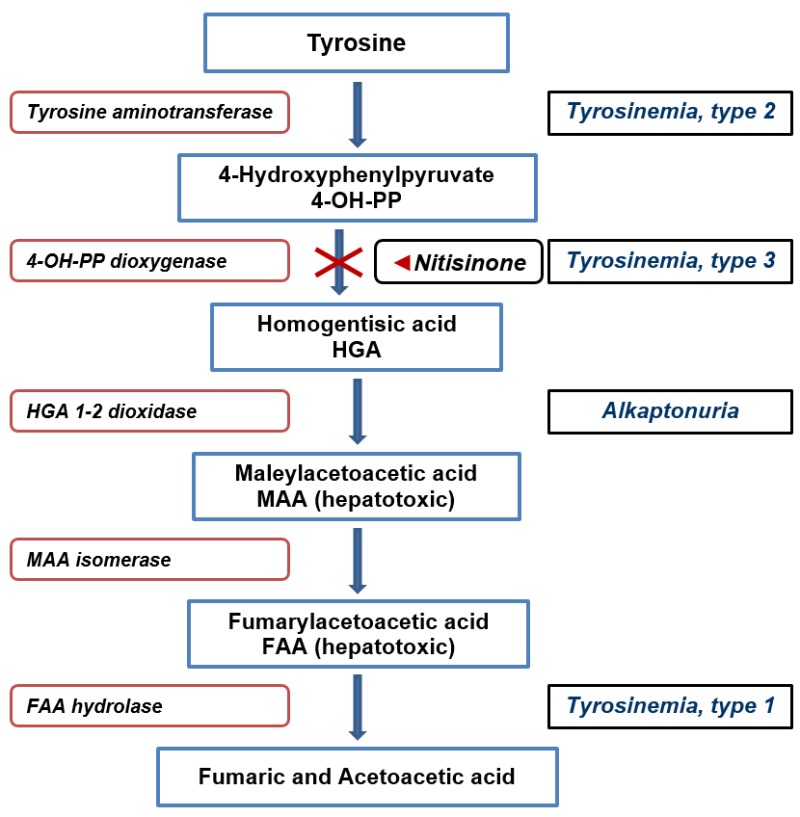
Hepatotoxicity
Type 1 tyrosinemia is a rare disease and clinical experience with use of nitisinone is limited. Therapy can be accompanied by mild elevations in serum aminotransferase levels, but these are generally mild (less than 3 times the upper limit of normal [ULN]) and often resolve even without dose modification. The aminotransferase elevations are not accompanied by symptoms or increases in serum alkaline phosphatase or bilirubin levels and rarely require dose modification. There have been no reports of clinically apparent liver injury attributed to nitisinone in the treatment of tyrosinemia or in experimental studies of its use in other disorders of tyrosine metabolism such as alkaptonuria.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which nitisinone causes serum aminotransferase elevations is unclear. Increased intrahepatic levels of tyrosine or 4-hydroxyphenylacetate may have mild toxic effects on hepatocytes or other tissues leading to mild and transient aminotransferase release.
Drug Class: Genetic Disorder Agents (Liver Diseases), Enzyme Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nitisinone – Orfadin®
DRUG CLASS
Genetic Disorder Agents
Product labeling at DailyMed, National Library of Medicine, NIH
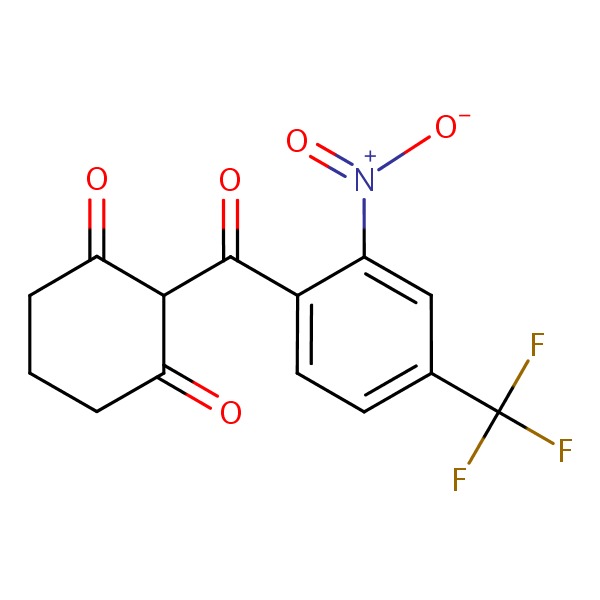
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nitisinone | 104206-65-7 | C14-H10-F3-N-O5 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 05 October 2016
- Zimmerman HJ. Bile acid derivatives. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721.(Expert review of hepatotoxicity published in 1999 before the availability of nitisinone).
- Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux: anti-emetics; agents used in biliary and pancreatic disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1323-50.(Textbook of pharmacology and therapeutics).
- Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 1992; 340 (8823): 813-7. [PubMed: 1383656](Initial report on use of nitisinone in humans; 5 children with type 1 tyrosinemia received nitisinone in doses of 0.1-0.6 mg/kg daily which led to clinical improvements in all including resolution of liver and renal abnormalities; “no side effects were encountered”).
- New treatment for tyrosinaemia. Lancet 1992; 340 (8823): 822-3. [PubMed: 1357248](Editorial in response to report by Lindstedt [1992] discusses the biologic basis for inhibition of tyrosine metabolism and the likelihood that nitisinone could greatly improve quality of life and life expectancy in type 1 tyrosinemia “if these results are confirmed”).
- Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet 1995; 10 (4): 453-60. [PubMed: 7545495](Description of an animal model of type 1 tyrosinemia, mice homozygous for fumarylacetoacetate hydrolase [FAH] deletion, and the effects of early therapy with nitisinone in reversing and preventing hepatic abnormalities including liver cancer).
- Pronicka E, Rowinska E, Bentkowski Z, Zawadzki J, Holme E, Lindstedt S. Treatment of two children with hereditary tyrosinaemia type I and long-standing renal disease with a 4-hydroxyphenylpyruvate dioxygenase inhibitor (NTBC). J Inherit Metab Dis 1996; 19: 234-8. [PubMed: 8739974](Two children with type 1 tyrosinemia and a chronic course marked mainly by renal rickets were treated with nitisinone and both had remission in renal disease with reversal of phosphate wasting, aminoaciduria and glycosuria).
- Lock EA, Ellis MK, Gaskin P, Robinson M, Auton TR, Provan WM, Smith LL, et al. From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis 1998; 21: 498-506. [PubMed: 9728330](History of development of nitisinone [NTBC], initially as an herbicide, but after demonstration of inhibition of tyrosine metabolism in animals, as a potential therapy of type 1 tyrosinemia [Lindstedt 1992]).
- Ros J, Vilaseca MA, Lambruschini N, Mas A, Lindstedt S, Holme E. NTBC as palliative treatment in chronic tyrosinaemia type I. J Inherit Metab Dis 1999; 22: 665-6. [PubMed: 10399099](A 17 year old boy with a long term history of liver disease, renal tubular dysfunction and severe osteomalaica was diagnosed with type 1 tyrosinemia and, despite the advanced nature of the disease, had a clinical response to dietary restriction and nitisinone, but died two years later with suspected liver cancer and respiratory failure).
- Crone J, Möslinger D, Bodamer OA, Schima W, Huber WD, Holme E, Stöckler Ipsiroglu S. Reversibility of cirrhotic regenerative liver nodules upon NTBC treatment in a child with tyrosinaemia type I. Acta Paediatr 2003; 92 (5): 625-8. [PubMed: 12839296](4 week old boy with acute tyrosinemia, type 1, was treated with nitisinone and low tyrosine diet and had reversal of liver failure and subsequent normal growth and development).
- Joshi SN, Venugopalan P. Experience with NTBC therapy in hereditary tyrosinaemia type I: an alternative to liver transplantation. Ann Trop Paediatr 2004; 24: 259-65. [PubMed: 15479577](5 infants with acute onset type 1 tyrosinemia started nitisinone therapy after 6-30 months of life and showed remarkable improvement within 2-6 months; therapy was “well tolerated without side-effects”).
- Suwannarat P, O'Brien K, Perry MB, Sebring N, Bernardini I, Kaiser-Kupfer MI, Rubin BI, et al. Use of nitisinone in patients with alkaptonuria. Metabolism 2005; 54: 719-28. [PubMed: 15931605](In a pilot study of nitisinone in 9 adults with alkaptonuria, all had a prompt and marked decrease in urinary homogentisic acid levels and most had improvements in arthritic pain, while side effects included ten-fold increases in tyrosine levels and mild increases in ALT levels [without increases in Alk P or bilirubin] in 5 patients [peak ALT 144 U/L], but abnormalities were asymptomatic and resolved within 1-3 weeks in 2 who stopped therapy).
- Masurel-Paulet A, Poggi-Bach J, Rolland MO, Bernard O, Guffon N, Dobbelaere D, Sarles J, et al. NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis 2008; 31: 81-7. [PubMed: 18214711](Among 46 French patients with type 1 tyrosinemia treated with a low protein diet and nitisinone for 3 months to 12 years [mean=4 years: dose 0.5-1.7 mg/kg daily], 4 required liver transplantation and two developed liver cancer, but most had a long term beneficial outcome; liver enzyme elevations during therapy were rare, almost all less than twice ULN and all were asymptomatic and resolved spontaneously).
- McKiernan PJ. Nitisinone in the treatment of hereditary tyrosinaemia type 1. Drugs 2006; 66 (6): 743-50. [PubMed: 16706549](Review of efficacy and safety of nitisinone in type 1 tyrosinemia, which results in resolution of liver failure in up to 90% of patients and improves renal tubular dysfunction as well, while adverse events are not common but can include marked elevations in serum tyrosine levels with possible corneal irritation and ulceration; no mention of liver injury).
- El-Karaksy H, Fahmy M, El-Raziky M, El-Koofy N, El-Sayed R, Rashed MS, El-Kiki H, El-Hennawy A, Mohsen N. Hereditary tyrosinemia type 1 from a single center in Egypt: clinical study of 22 cases. World J Pediatr 2011; 7: 224-31. [PubMed: 21633861](Among 4 Egyptian patients with type 1 tyrosinemia treated with nitisinone for 1-2 years, all had a clinical response and most were treated with less than 1 mg/kg daily, suggesting that lower doses may be as effective as the recommended doses of 1-2 mg/kg daily).
- Introne WJ, Perry MB, Troendle J, Tsilou E, Kayser MA, Suwannarat P, O'Brien KE, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab 2011; 103: 307-14. [PMC free article: PMC3148330] [PubMed: 21620748](Among 40 patients with alkaptonuria treated with nitisinone [2 mg daily] or no therapy for 3-4 years, homogentisic acid levels decreased into the normal range in all treated subjects, but arthritic symptoms or signs did not improve more with nitisinone compared to placebo therapy and adverse events included corneal opacities in one and fluctuating ALT elevations in another patient, with a peak level of 225 U/L which resolved upon stopping).
- Couce ML, Dalmau J, del Toro M, Pintos-Morell G, Aldámiz-Echevarría L; Spanish Working Group on Tyrosinemia type 1. Tyrosinemia type 1 in Spain: mutational analysis, treatment and long-term outcome. Pediatr Int 2011; 53: 985-9. [PubMed: 21752152](Among 34 Spanish patients with type 1 tyrosinemia treated with nitisinone [mean dose 0.9 mg/kg daily], only 1 patient required liver transplantation and none developed liver cancer).
- Wisse RP, Wittebol-Post D, Visser G, van der Lelij A. Corneal depositions in tyrosinaemia type I during treatment with nitisinone. BMJ Case Rep 2012; 2012. [PMC free article: PMC4543320] [PubMed: 23203167](A 17 year old boy with type 1 tyrosinemia treated with nitisinone [70 mg daily; duration not provided] developed corneal irritation and deposits which were suspected to be due to high plasma tyrosine levels, but actual values and outcome were not provided).
- Larochelle J, Alvarez F, Bussières JF, Chevalier I, Dallaire L, Dubois J, Faucher F, et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol Genet Metab 2012; 107: 49-54. [PubMed: 22885033](Retrospective analysis of 78 patients from Quebec with type 1 tyrosinemia seen between 1984 and 2004 found that 20 of 28 [71%] who never received nitisinone ultimately required liver transplantation compared to 7 of 26 [28%] who were treated late and none of 24 who started therapy early [before 30 days of age]; ALT elevations [above 60 U/L] occurred in 12 of 50 treated subjects [24%], but all were mild and transient [peak values 60-580 U/L] and did not require dose modification; no patient treated early developed liver disease or acute neurologic crisis).
- Schlune A, Thimm E, Herebian D, Spiekerkoetter U. Single dose NTBC-treatment of hereditary tyrosinemia type I. J Inherit Metab Dis 2012; 35: 831-6. [PubMed: 22307209](Plasma nitisinone levels did not change in 7 patients with tyrosinemia who were switched from twice to once daily dosing and turnover studies suggested a 54 hour half-life, indicating that once daily dosing might be optimal for routine, maintenance use).
- Thimm E, Richter-Werkle R, Kamp G, Molke B, Herebian D, Klee D, Mayatepek E, et al. Neurocognitive outcome in patients with hypertyrosinemia type I after long-term treatment with NTBC. J Inherit Metab Dis 2012; 35: 263-8. [PubMed: 22069142](Among 9 children with type 1 tyrosinemia treated since infancy with nitisinone, cognitive, neuromuscular and language development were below average in most and plasma tyrosine levels were high in all [12 month medians 403-804 µmol/L], suggesting that abnormal intellectual development is a feature of tyrosinemia and may be exacerbated by nitisinone therapy and the resultant, chronic elevation in tyrosine plasma levels).
- Vanclooster A, Devlieger R, Meersseman W, Spraul A, Kerckhove KV, Vermeersch P, Meulemans A, et al. Pregnancy during nitisinone treatment for tyrosinaemia type I: first human experience. JIMD Rep 2012; 5: 27-33. [PMC free article: PMC3509920] [PubMed: 23430914](A 19 year old woman with tyrosinemia had a normal pregnancy and newborn despite continuation of nitisinone therapy during pregnancy [0.5 mg/kg daily]).
- Bartlett DC, Lloyd C, McKiernan PJ, Newsome PN. Early nitisinone treatment reduces the need for liver transplantation in children with tyrosinaemia type 1 and improves post-transplant renal function. J Inherit Metab Dis 2014; 37: 745-52. [PubMed: 24515874](Retrospective analysis of 38 patients with tyrosinemia followed between 1989 and 2009 at a UK referral center found the rate of liver transplantation fell from 86% before 1992 compared to 23% thereafter, and children starting nitisinone early were least likely to ultimately require transplantation).
- Bendadi F, de Koning TJ, Visser G, Prinsen HC, de Sain MG, Verhoeven-Duif N, Sinnema G, et al. Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J Pediatr 2014; 164: 398-401. [PubMed: 24238861](Cross sectional study of 10 patients with type 1 tyrosinemia and their healthy siblings found lower IQs in patients vs controls [mean=71 vs 91] and serial testing in 5 patients found declines in IQ during nitisinone therapy, the impaired cognitive function being attributed to high plasma tyrosine levels [above 1500 µmol/L, normal 40-90 µmol/L]).
- Mayorandan S, Meyer U, Gokcay G, Segarra NG, de Baulny HO, van Spronsen F, Zeman J, et al. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J Rare Dis. 2014; 9: 107. [PMC free article: PMC4347563] [PubMed: 25081276](Among 168 patients with type 1 tyrosinemia from 21 centers in Europe, Turkey and Israel, the average age at diagnosis was 12.9 months; 154 received nitisinone for an average of 7.6 years, dose averaged 1.7 mg/kg daily, usually in 2 divided doses daily, 29 [17%] had liver transplantation [72% for liver cancer]; side effects included eye pain or itching [6%], corneal crystals [3%], thrombocytopenia [5%], and leukopenia [2%]; no mention of ALT elevations or hepatotoxicity).
- Kassel R, Sprietsma L, Rudnick DA. Pregnancy in an NTBC-treated patient with hereditary tyrosinemia type I. J Pediatr Gastroenterol Nutr 2015; 60: e5-7. [PubMed: 23838819](A 16 year old girl with tyrosinemia on long term nitisinone therapy became pregnant and delivered a normal, full term infant despite continuing treatment during entire pregnancy; tyrosine levels were elevated in the newborn, but rapidly fell into the normal range).
- Gertsman I, Barshop BA, Panyard-Davis J, Gangoiti JA, Nyhan WL. Metabolic effects of increasing doses of nitisinone in the treatment of alkaptonuria. JIMD Rep 2015; 24: 13-20. [PMC free article: PMC4582031] [PubMed: 25665838](Among 7 patients with alkaptonuria treated with escalating doses of nitisinone, urinary homogentisic acid levels decreased with increasing doses from 2-4 mg daily while serum tyrosine levels remained constant, suggesting that previous studies in alkaptonuria had used suboptimal doses [Introne 2011]).
- Zeybek AC, Kiykim E, Soyucen E, Cansever S, Altay S, Zubarioglu T, Erkan T, et al. Hereditary tyrosinemia type 1 in Turkey: twenty year single-center experience. Pediatr Int 2015; 57: 281-9. [PubMed: 25223216](Among 38 Turkish patients with type 1 tyrosinemia seen at a single referral center over a 20 year period, 36 were treated with nitisinone of whom 6 ultimately underwent liver transplantation, 4 for suspected liver cancer and 2 for noncompliance, and “no adverse effect required interruption of nitisinone treatment”, but no mention of ALT elevations).
- Simoncelli M, Samson J, Bussières JF, Lacroix J, Dorais M, Battista R, Perreault S. Cost-consequence analysis of nitisinone for treatment of tyrosinemia type I. Can J Hosp Pharm 2015; 68: 210-7. [PMC free article: PMC4485508] [PubMed: 26157182](Economic analysis concludes that nitisinone therapy decreases health care costs and is cost effective despite yearly price of more than $50,000).
- Ranganath LR, Milan AM, Hughes AT, Dutton JJ, Fitzgerald R, Briggs MC, Bygott H, et al. Suitability Of Nitisinone In Alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis 2016; 75: 362-7. [PubMed: 25475116](Among 40 patients with alkaptonuria treated with nitisinone [1, 2, 4 or 8 mg daily] or placebo for 4 weeks, urinary homogentisic acid levels fell in a dose related manner, the most efficacious dose being 8 mg daily [99% decrease] while plasma tyrosine levels were elevated at all doses, ranging from 400-1117 µmol/L and there were “no changes in clinical chemistry” results).
- Das AM. Pharmacotherapy of inborn errors of metabolism illustrating challenges in orphan diseases. J Pharmacol Toxicol Methods 2016; 81: 9-14. [PubMed: 26921514](Review of pharmacologic approaches to therapy of inborn errors of metabolism using substrate reduction, vitamins and cofactors, enzyme replacement or augmentation and transplantation, includes discussion of phenylketonuria, tyrosinemia, Gaucher disease and the urea cycle disorders).
- Gokay S, Ustkoyuncu PS, Kardas F, Kendirci M. The outcome of seven patients with hereditary tyrosinemia type 1. J Pediatr Endocrinol Metab. 2016 Sep 28. [Epub ahead of print] [PubMed: 27682708](Among 7 Turkish children with type 1 tyrosinemia who were treated with nitisinone, all had a clinical response, but one noncompliant child developed hepatocellular carcinoma at 10 years of age; no adverse events required discontinuation, but 2 children developed corneal opacities that resolved with strict adherence to low protein diet).
- In brief: Nitisinone (Orfadin) for hereditary tyrosinemia. Med Lett Drugs Ther 2016; 58 (1505): e132. [PubMed: 27701365](Short review of the efficacy, safety and costs of a new oral suspension formulation of nitisinone).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Clinical Review Report: Nitisinone (MDK-Nitisinone): (MendeliKABS Inc.): Indication: For the treatment of patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine[ 2018]Review Clinical Review Report: Nitisinone (MDK-Nitisinone): (MendeliKABS Inc.): Indication: For the treatment of patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine. 2018 Apr
- Review Clinical utility of nitisinone for the treatment of hereditary tyrosinemia type-1 (HT-1).[Appl Clin Genet. 2017]Review Clinical utility of nitisinone for the treatment of hereditary tyrosinemia type-1 (HT-1).Das AM. Appl Clin Genet. 2017; 10:43-48. Epub 2017 Jul 24.
- Review Clinical Review Report: Nitisinone (Nitisinone Tablets): (Cycle Pharmaceuticals Ltd.): Indication: For the treatment of patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine[ 2018]Review Clinical Review Report: Nitisinone (Nitisinone Tablets): (Cycle Pharmaceuticals Ltd.): Indication: For the treatment of patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine. 2018 Aug
- Phenotype, genotype, and outcome of 25 Palestinian patients with hereditary tyrosinemia type 1.[Metabol Open. 2021]Phenotype, genotype, and outcome of 25 Palestinian patients with hereditary tyrosinemia type 1.Dweikat I, Qawasmi N, Najeeb A, Radwan M. Metabol Open. 2021 Mar; 9:100083. Epub 2021 Jan 28.
- Review Clinical Review Report: Nitisinone (Orfadin): (Sobi Canada Inc.): Indication: For the treatment of patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine[ 2018]Review Clinical Review Report: Nitisinone (Orfadin): (Sobi Canada Inc.): Indication: For the treatment of patients with hereditary tyrosinemia type 1 in combination with dietary restriction of tyrosine and phenylalanine. 2018 Apr
- Nitisinone - LiverToxNitisinone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...