NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Naldemedine is a peripherally acting opioid antagonist which is used to treat constipation caused by chronic opioid use. Naldemedine has not been linked to serum enzyme elevations during therapy or to clinically apparent liver injury.
Background
Naldemedine (nal dem' e deen) is a semisynthetic opiate receptor antagonist which is similar structurally to naltrexone and blocks mu receptors in the enteric nervous system of the gastrointestinal tract resulting in an inhibition of opioid induced slowing of peristalsis. Naldemedine has a large polar side chain that does not block its engagement with opioid receptors, but does prevent it from crossing the blood brain barrier. As a consequence, naldemedine reverses the peripheral but not the central nervous system effects of opiates, such as pain relief and euphoria. In large preregistration trials, naldemedine was found to increase spontaneous bowel movement frequency and reduce constipation related side effects of opiates used for analgesia in patients with chronic, non-cancer pain. Naldemedine was approved for this use in the United States in 2017 and is currently available as tablets of 0.2 mg under the brand name Symproic. The recommended dose regimen is 0.2 mg once daily. Side effects can include abdominal pain, diarrhea, nausea, flatulence, anxiety, restlessness and sweating. Rare but potentially severe adverse events include withdrawal symptoms, hypersensitivity reactions and gastrointestinal perforation. Naldemedine has minimal effects on constipation in persons not taking opioids.
Hepatotoxicity
Therapy with naldemedine has not been linked to serum enzyme elevations or to clinically apparent liver injury. In preregistration studies, liver test abnormalities arose in less than 1% of treated patients but were transient, mild and not associated with symptoms. There were no reported cases of liver injury with jaundice or symptoms. Since its approval and more widescale use, there have been no published reports of hepatotoxicity attributed to naldemedine.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which naldemedine might cause liver injury is not known. Naldemedine is extensively metabolized in the liver, largely by CYP 3A4 and is a substrate for P-glycoprotein. It is susceptible to drug-drug interactions with agents that induce or inhibition CYP 3A or P-glycoprotein activity. Most opioid antagonists appear to have little intrinsic hepatotoxicity. The absence of evidence of hepatotoxicity for naldemedine may be in part due to the low total daily dose (<1 mg).
Drug Classes: Gastrointestinal Agents, Cathartics and Laxatives; Opioid Antagonists; Substance Abuse Treatment Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Naldemedine – Symproic®
DRUG CLASS
Gastrointestinal Agents; Opioid Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Naldemedine | 916072-89-4 | C32-H34-N4-O6 |
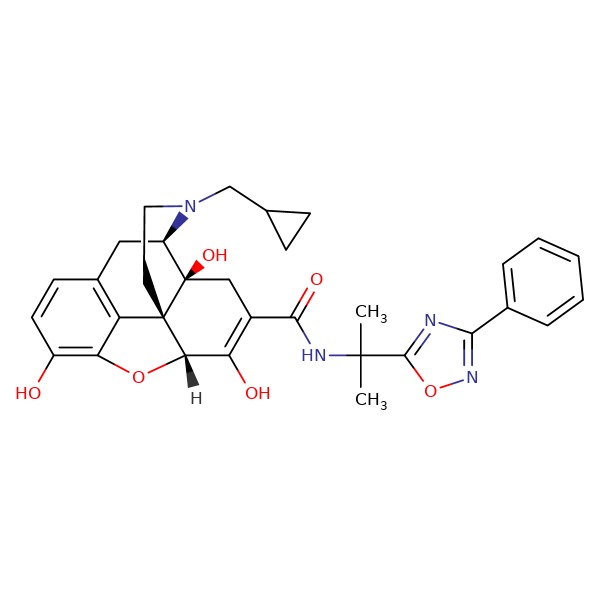 |
ANNOTATED BIBLIOGRAPHY
References updated: 15 April 2019
- Zimmerman HJ. Narcotic analgesics. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 710-11.(Expert review of hepatotoxicity published in 1999; mentions that trials of naltrexone have reported serum aminotransferase elevations in up to 30% of recipients, an effect that appeared to be partially dose dependent; naldemedine not discussed).
- Larrey D, Ripault MP. Illegal and recreational compounds. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 456-7.(Review of hepatotoxicity discusses buprenorphine, an orally available morphine analogue, which has been linked to cases of severe acute liver injury, usually as a result of intravenous administration; naldemedine is not discussed).
- Yaksh TL, Wallace MS. Opioids, analgesia, and pain management. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 355-86.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2017/208854Orig1s000MedR.pdf . (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; rates of liver test abnormalities were similar with naldemedine [0.8%: 9 of 1163] as placebo [0.8%: 9 of 1165] and no convincing case of clinically apparent liver injury could be attributed to naldemedine). - Webster LR, Yamada T, Arjona Ferreira JC. A phase 2b, randomized, double-blind placebo-controlled study to evaluate the efficacy and safety of naldemedine for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Med 2017; 18: 2350-60. [PMC free article: PMC5914456] [PubMed: 28371937](Among 244 patients with non-cancer pain and opioid induced constipation, naldemedine [0.1,0.2 or 0.4 mg] was associated with a higher, dose dependent response rate, while changes in chemistry laboratory results from baseline “were similar for all treatment groups”).
- Hale M, Wild J, Reddy J, Yamada T, Arjona Ferreira JC. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol 2017; 2: 555-64. [PubMed: 28576452](Among 1100 patients with opioid induced constipation enrolled in 2 placebo controlled 12 week trials, response rates were higher with naldemedine than placebo while adverse event rates were similar [22% vs 17%], diarrhea, nausea and abdominal pain being more frequent with naldemedine; no mention of ALT elevations or hepatotoxicity).
- Katakami N, Oda K, Tauchi K, Nakata K, Shinozaki K, Yokota T, Suzuki Y, et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol 2017; 35: 1921-8. [PubMed: 28445097](Among 227 patients with cancer and opioid induced constipation treated with naldemedine [0.1,0.2 or 0.4 mg] or placebo once daily for 14 days, response rates were higher with naldemedine and were dose related as were adverse event rates, diarrhea arising in 27%, 40% and 52% vs 25% in controls; no mention of ALT elevations or hepatotoxicity).
- Katakami N, Harada T, Murata T, Shinozaki K, Tsutsumi M, Yokota T, Arai M, et al. Randomized phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J Clin Oncol 2017; 35: 3859-66. [PubMed: 28968171](Among 193 patients with cancer and opioid induced constipation treated with naldemedine or placebo for 2 weeks followed by naldemedine for 12 weeks, clinical responses were more frequent with naldemedine [71% vs 34%] as were adverse events [44% vs 26%], and one developed a severe adverse event described as “abnormal hepatic function” but no details given).
- Markham A. Naldemedine: first global approval. Drugs 2017; 77: 923-7. [PubMed: 28466424](Summary of the structure, mechanism of action, pharmacology, clinical efficacy and safety of naldemedine; mentions that the most common adverse events are diarrhea, abdominal pain and nausea; no mention of ALT elevations or hepatotoxicity).
- Naldemedine (Symproic) for opioid-induced constipation. Med Lett Drugs Ther 2017; 9 (1535): 196-8. [PubMed: 29186083](Concise review of the mechanism of action, clinical efficacy, safety and costs of naldemedine shortly after its approval in the US for opioid induced constipation; mentions adverse events of abdominal pain, nausea and diarrhea as well as hypersensitivity reactions, withdrawal symptoms and gastrointestinal perforation, but does not mention ALT elevations or hepatotoxicity).
- Webster LR, Nalamachu S, Morlion B, Reddy J, Baba Y, Yamada T, Arjona Ferreira JC. Long-term use of naldemedine in the treatment of opioid-induced constipation in patients with chronic noncancer pain: a randomized, double-blind, placebo-controlled phase 3 study. Pain 2018; 159: 987-94. [PMC free article: PMC5916485] [PubMed: 29419653](Among 1246 patients with non-cancer pain and opioid induced constipation, overall adverse event rates were similar with naldemedine- [68%] as placebo-treatment [72%], while diarrhea was more frequent [11% vs 5%]; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Pharmacological Profile of Naldemedine, a Peripherally Acting μ-Opioid Receptor Antagonist: Comparison with Naloxone and Naloxegol.[J Pharmacol Exp Ther. 2020]Pharmacological Profile of Naldemedine, a Peripherally Acting μ-Opioid Receptor Antagonist: Comparison with Naloxone and Naloxegol.Kanemasa T, Koike K, Takase K, Arai T, Nakamura A, Morioka Y, Hasegawa M. J Pharmacol Exp Ther. 2020 Jun; 373(3):438-444. Epub 2020 Mar 13.
- Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials.[Lancet Gastroenterol Hepatol. ...]Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials.Hale M, Wild J, Reddy J, Yamada T, Arjona Ferreira JC. Lancet Gastroenterol Hepatol. 2017 Aug; 2(8):555-564. Epub 2017 May 30.
- Pharmacologic effects of naldemedine, a peripherally acting μ-opioid receptor antagonist, in in vitro and in vivo models of opioid-induced constipation.[Neurogastroenterol Motil. 2019]Pharmacologic effects of naldemedine, a peripherally acting μ-opioid receptor antagonist, in in vitro and in vivo models of opioid-induced constipation.Kanemasa T, Koike K, Arai T, Ono H, Horita N, Chiba H, Nakamura A, Morioka Y, Kihara T, Hasegawa M. Neurogastroenterol Motil. 2019 May; 31(5):e13563. Epub 2019 Feb 28.
- Review Naldemedine: A Review in Opioid-Induced Constipation.[Drugs. 2019]Review Naldemedine: A Review in Opioid-Induced Constipation.Blair HA. Drugs. 2019 Jul; 79(11):1241-1247.
- Review Update on the role of naldemedine in opioid-induced constipation in patients with chronic noncancer pain.[Therap Adv Gastroenterol. 2022]Review Update on the role of naldemedine in opioid-induced constipation in patients with chronic noncancer pain.BouSaba J, Sannaa W, Camilleri M. Therap Adv Gastroenterol. 2022; 15:17562848221078638. Epub 2022 Apr 28.
- Naldemedine - LiverToxNaldemedine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...