NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Miglitol is an alpha glucosidase inhibitor which delays intestinal absorption of carbohydrates and is used as an adjunctive therapy in the management of type 2 diabetes. Miglitol has not been linked to instances of clinically apparent acute liver injury.
Background
Miglitol is an inhibitor of intestinal alpha glucosidase which results in a decrease or delay in intestinal absorption of starch, disaccharides and dextrin. Miglitol is a deoxynojirimycin derivative similar in structure to glucose that inhibits alpha glucosidase activity in the intestinal brush border, blocking the breakdown of starch and disaccharides to absorbable monosaccharides and leading to a delay in carbohydrate absorption and thus blunting of the postprandial rise in blood glucose. Miglitol was approved for use in the United States in 1996 and was the second α-glucosidase inhibitor (after acarbose) introduced into clinical practice. The current indications are for management of glycemic control in type 2 diabetes used with diet and exercise alone or in combination with other oral hypoglycemic agents or insulin. Miglitol is available generically and under the brand name Glyset in tablets of 25, 50 and 100 mg. The typical initial dose in adults is 25 mg with each meal (with the first bite) followed by a gradual increase to a maximum of 100 mg three times daily. Miglitol causes malabsorption and gastrointestinal side effects are most common and flatulence, diarrhea, abdominal boating and rash. Severe adverse events can include rash and pneumatosis cystoides intestinalis. Hypoglycemia may occur with combinations of miglitol with other oral hypoglycemic agents.
Hepatotoxicity
In several large clinical trials, serum aminotransferase elevations were no more common with miglitol than with placebo, and all elevations that occurred were asymptomatic and resolved rapidly with stopping therapy. Neither during these studies nor since approval and wide clinical use have reports of clinically apparent liver injury due to miglitol been published. Thus, liver injury from miglitol must be very rare if it occurs at all. There also have been no reports of patients who developed liver injury while on acarbose being switched to miglitol.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The reason why miglitol rarely causes liver injury is not known. Unlike acarbose, miglitol is absorbed orally, but it is rapidly cleared by the kidneys and has minimal hepatic metabolism. While similar to acarbose (which does cause liver injury) in its mechanism of action, miglitol is a structurally distinct.
Drug Class: Antidiabetic Agents
Other Drugs in the Subclass Alpha Glucosidase Inhibitors: Acarbose
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Miglitol – Generic, Glyset®
DRUG CLASS
Antidiabetic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
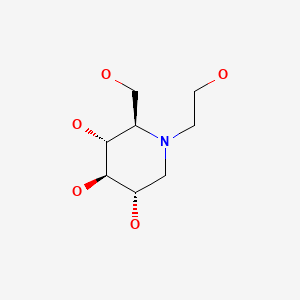
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Miglitol | 72432-03-2 | C8-H17-N-O5 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 12 January 2021
- Zimmerman HJ. Oral hypoglycemic agents and other diabetes therapy. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 575-9.(Textbook of hepatotoxicity published in 1999 mentions that several instances of serum enzyme elevations and at least two cases of liver injury with jaundice have been linked to acarbose use; no mention of miglitol).
- Bhardwaj SS, Chalasani NP. Antidiabetic drugs. Cardiovascular and antidiabetic medications. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 611-7.(Review of hepatotoxicity published in 2007 mentions that acarbose has been associated with hepatocellular injury despite the fact that it is minimally absorbed, but that miglitol has not been implicated in causing liver injury).
- Powers AC, D’Alessio D. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycemia. In, Brunton LL, Hillal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 863-86.(Textbook of pharmacology and therapeutics).
- Segal P, Feig PU, Schernthaner G, Ratzmann KP, Rybka J, Petzinna D, Berlin C. The efficacy and safety of miglitol therapy compared with glibenclamide in patients with NIDDM inadequately controlled by diet alone. Diabetes Care. 1997;20:687–91. [PubMed: 9135927](Controlled trial of miglitol vs glyburide vs placebo for 24 weeks in 201 patients with diabetes; 4 patients had elevations of liver enzymes at the end of the study [1 on miglitol, 1 glyburide and 2 placebo], but all 4 had some degree of elevation before treatment initiation).
- Miglitol for type 2 diabetes mellitus. Med Lett Drugs Ther. 1999;41(1053):49–50. [PubMed: 10368700](Brief review of role of miglitol in type 2 diabetes; "increased aminotransferase activity has not been reported with miglitol"; otherwise, the average cost, efficacy and tolerance of miglitol are similar to acarbose).
- Sels JP, Huijberts MS, Wolffenbuttel BH. Miglitol, a new alpha-glucosidase inhibitor. Expert Opin Pharmacother. 1999;1:149–56. [PubMed: 11249557](Review of the mechanism of action of miglitol and summary of results of clinical trials; no discussion of side effects).
- Campbell LK, Baker DE, Campbell RK. Miglitol: assessment of its role in the treatment of patients with diabetes mellitus. Ann Pharmacother. 2000;34:1291–301. [PubMed: 11098345](Systematic review of miglitol therapy of diabetes; rates of serum enzyme elevations during therapy with miglitol were low and similar to placebo).
- Scott LJ, Spencer CM. Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs. 2000;59:521–49. [PubMed: 10776834](Systematic review of literature on miglitol; side effects of flatulence [15-24%], diarrhea [7-13%], and abdominal pain [1-3%] were common, partially dose related, and similar to rates with acarbose; "Unlike acarbose, treatment with miglitol was not associated with elevated serum transaminase levels").
- Carlson RF. Miglitol and hepatotoxicity in type 2 diabetes mellitus. Am Fam Physician. 2000;62(2):315–318. [PubMed: 10929699](Letter in response to a review article on treatment of diabetes correcting the statement that miglitol causes liver injury similar to that described with acarbose; in studies for up to 12 months, ALT elevations above 3 times the ULN occurred in 3% of acarbose-, but only 1% of miglitol treated patients with diabetes).
- Chiasson JL, Naditch L., Miglitol Canadian University Investigator Group. The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care. 2001;24:989–94. [PubMed: 11375358](Controlled trial of 8 weeks of miglitol vs placebo with or without metformin in 324 patients with diabetes; there were no differences in biochemical test results among the treatment groups and no liver related serious adverse event).
- Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22:169–83. [PubMed: 12016548](Overview of hepatotoxicity of antidiabetic medications mentions that acarbose has been incriminated in hepatotoxicity, generally arising within 2-8 months of starting therapy with an acute hepatitis-like clinical picture, but that miglitol has not).
- van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–63. [PubMed: 15616251](Review of 41 studies, 30 of acarbose, 7 miglitol, 1 voglibose and 3 combined; discusses relative rates of side effects in comparison to placebo overall, but does not mention hepatic effects specifically).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver injury in the US collected from 2004 to 2008, none were attributed to miglitol or acarbose).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury, of which 4 were due to troglitazone, but none were attributed to miglitol or other antidiabetic medications).
- Drugs for type 2 diabetes. Treat Guidel Med Lett. 2011;9(108):47–54. [PubMed: 21778966](Concise review of the role of current antidiabetes medications in management of type 2 diabetes).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed antidiabetic agents including miglitol).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to an antidiabetic agent including miglitol).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, four were attributed to antidiabetic agents, including metformin, sitagliptin and glibenclamide, but none specifically to miglitol).
- Yokoh H, Kobayashi K, Sato Y, Takemoto M, Uchida D, Kanatsuka A, Kuribayashi N, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin compared with alpha-glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on metformin or pioglitazone alone (Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin-1): A multicenter, randomized, open-label, non-inferiority trial. J Diabetes Investig. 2015;6:182–91. [PMC free article: PMC4364853] [PubMed: 25802726](Among 119 Japanese adults with type 2 diabetes incompletely controlled on stable doses of oral agents who had the addition of sitagliptin or miglitol, reductions in HbA1c levels were less with miglitol while adverse events were more common [40% vs 10%] and mostly gastrointestinal; no mention of ALT elevations or hepatotoxicity).
- Komatsu M, Tanaka N, Kimura T, Fujimori N, Sano K, Horiuchi A, Sugiura A, et al. Miglitol attenuates non-alcoholic steatohepatitis in diabetic patients. Hepatol Res. 2018;48:1092–8. [PubMed: 29935004](Among 17 adults with type 2 diabetes and nonalcoholic steatohepatitis treated with miglitol for 12 months, ALT and HbA1c levels and body weight decreased overall as did liver biopsy scores for steatosis and inflammation but not for fibrosis or ballooning; there were no serious adverse events and no mention of ALT elevations or hepatotoxicity).
- Hedrington MS, Davis SN. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin Pharmacother. 2019;20:2229–35. [PubMed: 31593486](Review of the role of alpha glucosidase inhibitors in the therapy of type 2 diabetes mentions that side effects are largely gastrointestinal [diarrhea, abdominal pain, flatulence] and are transient and mostly dose dependent; no mention of ALT elevations or hepatotoxicity).
- Drugs for type 2 diabetes. Med Lett Drugs Ther. 2019;61(1584):169–78. [PubMed: 31770362](Concise review of the mechanisms of action, clinical efficacy, side effects and costs of currently available drugs for type 2 diabetes mentions that miglitol and acarbose must be taken with each meal and can lower HbA1c levels by 0.5-1.0%; side effects are not discussed).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Alpha Glucosidase Inhibitors.[LiverTox: Clinical and Researc...]Review Alpha Glucosidase Inhibitors.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Changes in α-glucosidase activities along the jejunal-ileal axis of normal rats by the α-glucosidase inhibitor miglitol.[Metabolism. 2010]Changes in α-glucosidase activities along the jejunal-ileal axis of normal rats by the α-glucosidase inhibitor miglitol.Mochizuki K, Hanai E, Suruga K, Kuranuki S, Goda T. Metabolism. 2010 Oct; 59(10):1442-7. Epub 2010 Feb 11.
- Review Acarbose.[LiverTox: Clinical and Researc...]Review Acarbose.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- [Delayed absorption of carbohydrates in the therapy of Type II diabetes: comparison between dietary (Muesli) and pharmacological (Alpha-glucosidase inhibition) modification].[Schweiz Med Wochenschr. 1991][Delayed absorption of carbohydrates in the therapy of Type II diabetes: comparison between dietary (Muesli) and pharmacological (Alpha-glucosidase inhibition) modification].Willms B, Lübke D, Ahrens K, Arends J. Schweiz Med Wochenschr. 1991 Sep 21; 121(38):1379-82.
- Review Miglitol: assessment of its role in the treatment of patients with diabetes mellitus.[Ann Pharmacother. 2000]Review Miglitol: assessment of its role in the treatment of patients with diabetes mellitus.Campbell LK, Baker DE, Campbell RK. Ann Pharmacother. 2000 Nov; 34(11):1291-301.
- Miglitol - LiverToxMiglitol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...