NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Methocarbamol is a commonly used, centrally acting muscle relaxant and has not been linked to instances of liver injury.
Background
Methocarbamol (meth" oh kar' ba mol) is a guaifenesin derivative and acts centrally as a muscle relaxant by an unknown mechanism. Methocarbamol was approved for use in the United States in 1957, and currently more than 3 million prescriptions are filled yearly. Methocarbamol is indicated for the relief of acute, painful musculoskeletal conditions. Methocarbamol is available in 500 and 750 mg tablets in several generic formulations, both alone and in combination with other drugs and under the brand names of Robaxin and Marbaxin. The recommended dosage is 1500 mg orally three to four times daily. The most common side effects of methocarbamol are drowsiness blurred vision, headache, nausea and skin rash.
Hepatotoxicity
While the product label for methocarbamol states that it can cause jaundice (including cholestatic jaundice), there is little published evidence to suggest that methocarbamol is a cause of hepatic injury or clinically apparent drug induced liver disease. During clinical trials of methocarbamol, some patients had to stop treatment because of nausea, dizziness, or other nonspecific complaints, but no serum aminotransferase levels or other laboratory results were reported. Methocarbamol appears to be well tolerated, but the lack of monitoring of serum aminotransferase levels during clinical trials with methocarbamol makes it impossible to rule out the possibility of mild liver injury occurring with treatment.
Likelihood score: E (Unlikely cause of clinically apparent liver injury).
Drug Class: Muscle Relaxants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Methocarbamol – Generic, Robaxin®
DRUG CLASS
Autonomic Agents: Muscle Relaxants, Central
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Methocarbamol | 532-03-6 | C11-H15-N-O5 |
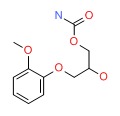 |
ANNOTATED BIBLIOGRAPHY
References updated: 30 January 2017
- Zimmerman HJ. Muscle spasmolytics. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 544-45.(Expert review of hepatotoxicity published in 1999; discussed dantrolene, chlorzoxazone and baclofen, but not methocaramol).
- Hibbs RE, Zambon AC. Agents acting at the neuromuscular junction and autonomic ganglia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011. p. 255-76.(Textbook of pharmacology and therapeutics).
- Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage 2004; 28: 140-75. [PubMed: 15276195](Thorough review of the pharmacology, efficacy and side effects of the muscle relaxants).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 [0.7%] were attributed to muscle relaxants, including dantrolene, baclofen, chlorzoxazone and metaxalone, but none were linked to use of methocarbamol).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- [Spasm and other painful conditions of skeletal muscles. Clinical investigation of the effectiveness and tolerance of the centrally-acting muscle relaxant methocarbamol].[Fortschr Med Suppl. 1992][Spasm and other painful conditions of skeletal muscles. Clinical investigation of the effectiveness and tolerance of the centrally-acting muscle relaxant methocarbamol].. Fortschr Med Suppl. 1992; 120:1-7.
- Pharmacological profile of N-(2,6-DIMETHYLPHENYL)-N-(1-methyl-2pyrrolidinylidene)urea, xilobam, a new centrally acting skeletal muscle relaxant.[Arch Int Pharmacodyn Ther. 1978]Pharmacological profile of N-(2,6-DIMETHYLPHENYL)-N-(1-methyl-2pyrrolidinylidene)urea, xilobam, a new centrally acting skeletal muscle relaxant.Gardocki JF, Hageman WE, Pruss TP. Arch Int Pharmacodyn Ther. 1978 Jun; 233(2):326-42.
- Muscle relaxant activity of methocarbamol enantiomers in mice.[J Pharm Pharmacol. 1999]Muscle relaxant activity of methocarbamol enantiomers in mice.Souri E, Sharifzadeh M, Farsam H, Gharavi N. J Pharm Pharmacol. 1999 Jul; 51(7):853-5.
- Review Baclofen.[LiverTox: Clinical and Researc...]Review Baclofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Chlorzoxazone.[LiverTox: Clinical and Researc...]Review Chlorzoxazone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Methocarbamol - LiverToxMethocarbamol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...