NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Melphalan is an orally and parenterally administered nitrogen mustard-like alkylating agent used in the therapy of multiple myeloma and ovarian cancer. Melphalan therapy has been associated with low rates of serum enzyme elevations during therapy, but when used in high doses as myeloablative therapy in preparation for hematopoietic cell transplantation, it is associated with high rates of enzyme elevations and acute liver injury due to sinusoidal obstruction syndrome.
Background
Melphalan (mel' fa lan) is a phenylalanine derivative of nitrogen mustard and an alkylating agent that resembles cyclophosphamide and chlorambucil. It acts by causing modification and cross linking of DNA, thus inhibiting DNA, RNA and protein synthesis and causing programmed cell death (apoptosis) in rapidly dividing cells. Melphalan was approved for use in the United States in 1964. Current indications for oral melphalan include multiple myeloma and some forms of ovarian carcinoma, usually in combination with other antineoplastic agents. Melphalan is also used parenterally in myeloablative regimens in preparation for hematopoietic cell transplantation and experimentally in high doses in local-regional therapy of metastatic cancer. Melphalan is available as tablets of 2 mg and as a powder for intravenous use in vials of 50 mg generically and under the trade name of Alkeran. The recommended dose varies by indication, body weight and renal function. Melphalan is often given in cycles of 1 to 3 weeks, with 2 to 4 week intervals between cycles. Melphalan shares common side effects with nitrogen mustards and other alkylating agents; these side effects include nausea, vomiting, diarrhea, alopecia, pruritus, bone marrow suppression, oral ulceration, rash and hypersensitivity reactions. Uncommon, but potentially severe adverse reactions include irreversible myelosuppression, hemoloytic anemia, pulmonary fibrosis and interstitial pneumonitis, hepatotoxicity and anaphylaxis.
Hepatotoxicity
Mild and transient elevations in serum aminotransferase levels are uncommon with standard doses of melphalan, but occur more commonly with high dose intravenous regimens. The enzyme elevations are usually mild and asymptomatic and do not require dose modification. Clinically apparent liver disease has not been reported with standard doses of melphalan in chemotherapeutic regimens. However, use of high doses of melphalan in preparation for hematopoietic cell (bone marrow and stem cell) transplantation or in local-regional therapy has been associated with sinusoidal obstruction syndrome that can be severe, leading to acute liver failure and death. Onset of sinusoidal obstruction syndrome is usually within 1 to 3 weeks of the myeloablative or high dose therapy, and is characterized by the onset of abdominal pain, hepatomegaly, weight gain and ascites followed by jaundice. The pattern of serum enzyme elevations is usually hepatocellular, with marked increases in serum aminotransferase and lactic dehydrogenase levels and minimal increase in alkaline phosphatase. The severity of injury varies considerably. In mild forms there is serum enzyme elevations without jaundice and with few or no symptoms. In severe instances, there are elevations in prothrombin time and progressive jaundice and hepatic failure. Immunoallergic and autoimmune features are uncommon. The fatality rate is high. Liver biopsy shows centrolobular necrosis and congestion with occlusion of small veins and red cells in sinusoids.
Melphalan has also been used in the regional therapy of nonresectable cancers in a limb, solid organ or even the liver. In isolated, hyperthermic hepatic perfusion with chemotherapeutic agents, the liver is isolated surgically and perfused with melphalan, with or without tumor necrosis factor (or other antineoplastic agents). The perfusion typically lasts for at least 1 hour and results in a high rate of tumor regression, but with a high rate of hepatotoxicity. At least 80% of patients develop significant serum aminotransferase elevations (5 to 20 times ULN) and two-thirds develop jaundice transiently. The liver injury can be severe and even fatal if complicated by severe sinusoidal obstruction syndrome, direct cytotoxicity or hyperthermia or ischemic injury.
Finally, melphalan is a cytotoxic and immunosuppressive agent and has been linked to rare instances of reactivation of hepatitis B which can be severe and even fatal. Reactivation typically occurs in patients with inactive hepatitits B with HBsAg, but no HBeAg and no or only low levels of HBV DNA in serum before treatment. Reactivation is marked by sudden rise in HBV DNA levels and subsequent increases in ALT and, in severe instances, jaundice and signs of hepatic failure. Reactivation can also occur in patients who are HBsAg and HBV DNA negative, but have anti-HBc in serum with or without anti-HBs, a serologic pattern suggestive of recovery from hepatitis B. In this situation, referred to as “reverse seroconversion”, HBV DNA levels arise followed by HBsAg and serum enzyme elevations. Reverse seroconversion tends to be severe. Most cases of reactivation of hepatitis B attributed to cytotoxic alkylating agents such as melphalan have occurred in patients who are also receiving high doses of corticosteroids, which on their own can lead to reactivation. The combination of the two immunosuppressive agents is probably more likely to cause reactivation than either alone.
Likelihood score: B[H] (likely cause of clinically apparent liver injury when used in high doses).
Mechanism of Injury
The mechanism of hepatotoxicity from melphalan is probably direct cytotoxic injury to sinusoidal endothelial cells causing their death and extrusion into sinusoids, with subsequent obstruction of sinusoids and small hepatic veins. The cytotoxic and immunosuppressive activity of melphalan may also be responsible for the risk of reactivation of hepatitis B. Melphalan has minimal hepatic metabolism and has not been reported to have significant drug-drug interactions. Cases with features of idiosyncratic liver injury have not been described.
Outcome and Management
The severity of sinusoidal obstruction syndrome varies considerably, from transient mild asymptomatic liver injury to acute liver failure that is rapidly fatal. There is no known specific therapy of proven efficacy for sinusoidal obstruction syndrome and management should focus on avoidance of further injury and supportive care. Rechallenge should not be done. Reactivation of hepatitis B can be prevented by prophylactic therapy with antiviral agents active against HBV, such as tenofovir and entecavir. Patients with serious malignancies undergoing systemic chemotherapy, such as with melphalan, should be screened for HBsAg and anti-HBc before starting chemotherapy and offered prophylaxis if HBsAg positive and monitored closely if HBsAg negative but anti-HBc positive.
Drug Class: Antineoplastic Agents, Alkylating Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Melphalan – Generic, Alkeran®
DRUG CLASS
Antineoplastic Agents, Alkylating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Melphalan | 148-82-3 | C13-H18-Cl2-N2-O2 |
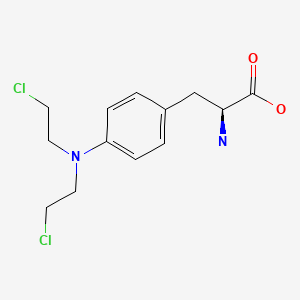
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 January 2020
Abbreviations used: SOS, sinusoidal obstruction syndrome; HCT, hematopoietic cell transplantation.
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that melphalan has not been linked to significant liver injury although high doses can cause serum enzyme elevations).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 551.(Review of hepatotoxicity of cancer chemotherapeutic agents mentions that melphalan has little or no effect on liver tests).
- Wellstein A, CGiaccone G, Atkins MB, Sausville EA. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1167-202.(Textbook of pharmacology and therapeutics).
- Singhal S, Powles R, Treleaven J, Horton C, Swansbury GJ, Mehta J. Melphalan alone prior to allogeneic bone marrow transplantation from HLA-identical sibling donors for hematologic malignancies: alloengraftment with potential preservation of fertility in women. Bone Marrow Transplant. 1996;18:1049–55. [PubMed: 8971372](28 patients undergoing bone marrow transplantation for leukemia or lymphoma received melphalan alone as conditioning agent; 9 [32%] died, but none of liver disease and no mention of veno-occlusive disease; 3 of 4 surviving women were able to bear children after therapy and transplantation).
- Rollins BJ. Hepatic veno-occlusive disease. Am J Med. 1986;8:297–306. [PubMed: 3526887](Review of the diagnosis, clinical course, histology and pathogenesis of veno-occlusive disease which is now referred to as sinusoidal obstruction syndrome [SOS]).
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, et al. Veno-occlusive disease of the liver following bone marrow transplantation. Transplantation. 1987;4:778–83. [PubMed: 3321587](Among 235 patients undergoing bone marrow transplantation between 1982 and 1985, SOS developed in 52 [22%] of whom half died, making SOS the third most common cause of death in this population).
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–20. [PubMed: 7756636](Review of hepatic SOS after bone marrow transplantation [now referred to as hematopoietic cell transplantation [HCT]; usually presents with painful hepatomegaly, weight gain [fluid and ascites] and jaundice within 3 weeks of myeloablation, with occlusion of central veins and sinusoids and extensive zone 3 [centrolobular] injury).
- Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BBR, Schlag PM, Lienard D, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas: the cumulative multicenter European experience. Ann Surg. 1996;224:756–64. [PMC free article: PMC1235474] [PubMed: 8968230](Among 186 patients with sarcoma undergoing isolated limb perfusion with tumor necrosis factor and melphalan, some systemic adverse events occur, perhaps because of leakage; ALT elevations above 5 times ULN occurred in 6 patients [3%] but no other hepatic adverse events mentioned).
- Moreau P, Fiere D, Bezwoda WR, Facon T, Attal M, Laporte JP, Colombat P, et al. Prospective randomized placebo-controlled study of granulocyte-macrophage colony-stimulating factor without stem-cell transplantation after high-dose melphalan in patients with multiple myeloma. J Clin Oncol. 1997;15:660–6. [PubMed: 9053491](Among 102 patients with multiple myeloma given high doses of melphalan, 20% had AST and 12% bilirubin elevations; no further details provided).
- Lee JL, Gooley T, Bensinger W, Schiffman K, McDonald GB. Veno-occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 1999;5:306–15. [PubMed: 10534061](Among 253 patients who received a regimen of busulfan, melphalan and thiotepa in preparation for HCT, 70 [28%] developed SOS, which was severe in 11 and fatal in 9 [4%]).
- Alexander HR, Libutti SK, Bartlett DL, Puhlmann M, Fraker DL, Bachenheimer LC. A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2000;6:3062–70. [PubMed: 10955785](Among 22 patients with melanoma metastatic to liver treated with isolated liver perfusion with hyperthermia, melphalan and tumor necrosis factor [TNF], 82% had ALT elevations [with or without TNF] and 68% had bilirubin elevations, 1 dying of multiorgan failure).
- Christoforidis D, Martinet O, Lejeune FJ, Mosimann F. Isolated liver perfusion for non-resectable liver tumours: a review. Eur J Surg Oncol. 2002;28:875–90. [PubMed: 12477481](Review of isolated liver perfusion with melphalan [with or without tumor necrosis factor] as therapy of nonresectable liver tumors; liver failure and deaths have been reported due to SOS, coagulopathy, tumor replacement of the liver and hyperthermia-ischemia injury; elevations of ALT to 5-20 times ULN is common as is jaundice with bilirubin levels of 3 to 10 mg/dL).
- Bouyn CI, Leclere J, Raimondo G, Le Pointe HD, Couanet D, Valteau-Couanet D, Hartmann O. Hepatic focal nodular hyperplasia in children previously treated for a solid tumor. Incidence, risk factors, and outcome. Cancer. 2003;97:3107–13. [PubMed: 12784348](Retrospective analysis of 14 cases of focal nodular hyperplasia identified in children who had been treated for malignancy [neuroblastoma, sarcoma, germ cell tumors] with busulfan [n=9], melphalan [n=9], radiation [n=6] and bone marrow transplantation [n=1] in the distant past [4-21 years], usually found incidentally by ultrasound and not associated with symptoms or liver test abnormalities).
- Kusumi E, Kami M, Kanda Y, Murashige N, Seki K, Fujiwara M, Koyama R, et al. Hepatic injury following reduced intensity unrelated cord blood transplantation for adult patients with hematological diseases. Biol Blood Marrow Transplant. 2006;12:1302–9. [PubMed: 17162212](Among 85 patients undergoing allogenic cord blood transplantation after ablative therapy with melphalan or busulfan combined with fludarabine and total body irradiation; 87% developed hyperbilirubinemia and 19% had ALT elevations >5 times ULN, the usual causes being graft-vs-host disease and sepsis).
- Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey J-N, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Brit J Surg. 2007;94:274–86. [PubMed: 17315288](Systematic review of liver toxicity occurring after preoperative systemic chemotherapy for colorectal liver metastases, focuses upon steatosis caused by irinotecan and many other agents, SOS from oxiplatin and sclerosing cholangitis from fluoxuridine).
- van Iersel LB, Verlaan MR, Vahrmeijer AL, van Persijn van Meerten EL, Tijl FG, Sparidans RW, Gelderblom H, et al. Hepatic artery infusion of high-dose melphalan at reduced flow during isolated hepatic perfusion for the treatment of colorectal metastases confined to the liver: a clinical and pharmacologic evaluation. Eur J Surg Oncol. 2007;33:874–81. [PubMed: 17400422](Among 30 patients with nonresectable liver metastases undergoing isolated hepatic perfusion with melphalan, most developed hepatic toxicity, 4 had sinusoidal obstruction syndrome, one died of liver failure and two developed portal hypertension).
- Carreras E, Rosinol L, Terol MJ, Alegre A, de Arriba F, Garcia-Larana J, Bello JL, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant. 2007;13:1448–54. [PubMed: 18022574](Among 734 patients undergoing autologous HCT for multiple myeloma, SOS occurred in 8% [19 of 240 with 2% mortality, median onset 29 days] receiving busulfan and melphalan, but in only 0.4% [2 of 494: 0.2% mortality, onset 9-13 days] receiving melphalan alone).
- Mocellin S, Pilati P, Da Pian P, Forlin M, Corazzina S, Rossi CR, Innocente F, et al. Correlation between melphalan pharmacokinetics and hepatic toxicity following hyperthermic isolated liver perfusion for unresectable metastatic disease. Ann Surg Oncol. 2007;14:802–9. [PubMed: 17103263](Among 20 patients with nonresectable liver metastases undergoing isolated 60 minute hepatic perfusions with hyperthermia and melphalan, all patients developed hepatic injury, which was severe in 55% and fatal in 15%; degree of hepatotoxicity correlated with higher melphalan perfusate levels but not with tumor response, melphalan dose, body weight or other clinical features).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, antineoplastic agents were rarely implicated: 3 were considered due to mercaptopurine and 1 each due to bortezombin, cyclophosphamide, docetaxel, and temozolomide).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but few anticancer drugs were implicated [1 case each for melphalan and gemtuzumab]).
- McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–60. [PMC free article: PMC2914093] [PubMed: 20373370](Review of liver complications of HCT, which have become less frequent with better understanding of their causes and means of prevention; the rate of SOS has decreased because of avoidance of more aggressive ablative therapies [total body irradiation and high doses of cyclophosphamide] and better understanding of pharmacokinetics of the alkylating agents).
- Yamamoto M, Zager JS. Isolated hepatic perfusion for metastatic melanoma. J Surg Oncol. 2014;109(4):383–8. [PubMed: 24166748](Review of the technique, efficacy and safety of isolated hepatic perfusion as therapy of metastatic melanoma, usually with melphalan with or without tumor necrosis factor as chemotherapy; no discussion of hepatotoxicity).
- Voron T, Zinzindohoué F, Journois D, Hervé C, Ponzio O, Lucas N. Hyperthermic isolated liver perfusion with melphalan and bevacizumab. J Visc Surg. 2013;150:60–6. [PubMed: 23182850](Two patients with unresectable liver cancers underwent isolated 1-hr, hyperthermic liver perfusion with melphalan and bevacizumab without evidence of hepatic failure, SOS or residual portal hypertension).
- Landau H, Hassoun H, Rosenzweig MA, Maurer M, Liu J, Flombaum C, Bello C, et al. Bortezomib and dexamethasone consolidation following risk-adapted melphalan and stem cell transplantation for patients with newly diagnosed light-chain amyloidosis. Leukemia. 2013;27:823–8. [PubMed: 23014566](Among 40 patients with amyloidosis who were treated with 3 doses of melphalan followed by HCT with or without consolidation with bortezomib and dexamethaxone, side effects were common, but no patient developed serious, clinically apparent hepatic toxicity).
- Reddy SK, Kesmodel SB, Alexander HR Jr. Isolated hepatic perfusion for patients with liver metastases. Ther Adv Med Oncol. 2014;6:180–94. [PMC free article: PMC4107710] [PubMed: 25057304](Review of isolated hepatic perfusion, including details of the procedure, clinical results and toxicity, mentions that the most common adverse event was ALT elevations [Grade III or above] occurring in 65% of patients).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, 10 were attributed to antineoplastic agents, but none to melphalan).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. PMID: 25754159. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5.5%] were attributed to antineoplastic agents, including 3 to alkylating agents, but none to melphalan).
- Laemmle A, Hahn D, Hu L, Rüfenacht V, Gautschi M, Leibundgut K, Nuoffer JM, et al. Fatal hyperammonemia and carbamoyl phosphate synthetase 1 (CPS1) deficiency following high-dose chemotherapy and autologous hematopoietic stem cell transplantation. Mol Genet Metab. 2015;114:438–44. [PubMed: 25639153](A 2 year old with neuroblastoma developed severe hyperammonemia 7 days after completing myeloablation with carboplatin, etoposide and melphalan followed by HCT [NH3 475 umol/L, lactate 9.2 mmol/L] despite normal bilirubin and liver tests and a liver biopsy showing “no further signs of liver disease”, biochemical testing showing marked decreases in urea cycle enzyme activity and levels despite normal gene sequences and mRNA levels suggesting impaired mitochondrial function).
- Moon WR, Moon DS, Kim J, Yoon YM, Choi BS, Chung CH, Park SG. Reactivation of hepatitis B (reverse seroconversion) after melphalan/dexamethasone therapy for primary amyloidosis: a case report. J Med Case Rep. 2015;9:122. [PMC free article: PMC4460636] [PubMed: 26031455](A 77 year old Korean man with primary amyloidosis developed reactivation of hepatitis B after 6 cycles of melphalan with dexamethasone [doses not given] despite being HBsAg negative and anti-HBs positive before therapy [bilirubin 1.3 mg/dL, ALT 496 U/L, HBsAg positive, HBeAg positive, HBV DNA ~ 67 million copies/mL], responding with clearance of HBsAg and normalization of ALT after entecavir therapy).
- Hughes MS, Zager J, Faries M, Alexander HR, Royal RE, Wood B, Choi J, et al. Results of a randomized controlled multicenter phase III trial of percutaneous hepatic perfusion compared with best available care for patients with melanoma liver metastases. Ann Surg Oncol. 2016;23:1309–19. [PMC free article: PMC8185532] [PubMed: 26597368](Among 93 patients with melanoma metastatic to the liver enrolled in a randomized controlled trial, overall survival was not different in those treated with percutaneous hepatic perfusion with melphalan vs standard therapy, although side effects were greater and 4 patients died of complications although none from liver injury which was scored as Grade III/IV in 14% of subjects).
- Kirstein MM, Marquardt S, Jedicke N, Marhenke S, Koppert W, Manns MP, Wacker F, et al. Safety and efficacy of chemosaturation in patients with primary and secondary liver tumors. J Cancer Res Clin Oncol. 2017;143:2113–21. [PubMed: 28634727](Among 29 patients treated with percutaneous hepatic perfusion with melphalan and hemofiltration of the hepatic venous outflow for various hepatic metastases or primary liver cancers, 19% had an objective response and major toxicities were largely hematologic and transient, ALT elevations rising above 5 times ULN in 17% generally in the week following the procedure and resolving in all).
- Vogel A, Gupta S, Zeile M, von Haken R, Brüning R, Lotz G, Vahrmeijer A, et al. Chemosaturation percutaneous hepatic perfusion: a systematic review. Adv Ther. 2017;33:2122–38. [PMC free article: PMC5126197] [PubMed: 27798773](Description of results using a commercial delivery system for percutaneous hepatic perfusion of melphalan for unresectable liver tumors or metastases which concluded that it had “favorable tumor response rates” and an “acceptable safety profile”).
- Vogl TJ, Koch SA, Lotz G, Gebauer B, Willinek W, Engelke C, Brüning R, et al. Percutaneous isolated hepatic perfusion as a treatment for isolated hepatic metastases of uveal melanoma: patient outcome and safety in a multi-centre study. Cardiovasc Intervent Radiol. 2017;40:864–72. [PubMed: 28144756](Among 18 patients with ocular melanoma and liver metastases treated with percutaneous isolated hepatic perfusion with melphalan, adverse events were frequent, including leukopenia, thrombocytopenia, fever and edema; no mention of ALT elevations or hepatotoxicity).
- Abate ME, Paioli A, Cammelli S, Cesari M, Longhi A, Palmerini E, Ferrari S, et al. Sinusoidal obstruction syndrome/veno-occlusive disease after high-dose intravenous busulfan/melphalan conditioning therapy in high-risk Ewing Sarcoma. Bone Marrow Transplant. 2018;53:591–9. [PubMed: 29335623](Among 75 patients with Ewing sarcoma treated with busulfan and melphalan followed by HCT, 5 developed moderate-to-severe SOS [7%], all arising in adults [5/43: 12%], mostly males [4/48: 8%] or with a history of radiation therapy [4/32: 13%]).
- Artzner C, Mossakowski O, Hefferman G, Grosse U, Hoffmann R, Forschner A, Eigentler T, et al. Chemosaturation with percutaneous hepatic perfusion of melphalan for liver-dominant metastatic uveal melanoma: a single center experience. Cancer Imaging. 2019;19:31. [PMC free article: PMC6543599] [PubMed: 31146793](Among 16 patients with uveal melanoma and liver metastases treated with 28 courses of percutaneous hepatic perfusion, a partial response occurred in 9 [60%] but adverse events were common, including “liver toxicity” in 46% of courses, which was mild [Grade I] in all).
- Meijer TS, Burgmans MC, Fiocco M, de Geus-Oei LF, Kapiteijn E, de Leede EM, Martini CH, et al. Safety of percutaneous hepatic perfusion with melphalan in patients with unresectable liver metastases from ocular melanoma using the Delcath Systems' second-generation hemofiltration system: a prospective non-randomized phase II trial. Cardiovasc Intervent Radiol. 2019;42:841–52. [PMC free article: PMC6502784] [PubMed: 30767147](Among 35 patients with ocular melanoma with liver metastases treated with 67 courses of percutaneous hepatic perfusion with melphalan using a new hemofiltration system, there were 14 serious mostly hematologic adverse events, none hepatic although aminotransferase elevations arose the majority of patients, all were mild and resolved within 16 days of the procedure).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Busulfan.[LiverTox: Clinical and Researc...]Review Busulfan.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study.[Lancet Haematol. 2020]Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study.Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, Dozza L, van der Holt B, Zweegman S, Oliva S, et al. Lancet Haematol. 2020 Jun; 7(6):e456-e468. Epub 2020 Apr 30.
- Propylene Glycol-Free Melphalan versus PG-Melphalan as Conditioning for Autologous Hematopoietic Cell Transplantation for Myeloma.[Biol Blood Marrow Transplant. ...]Propylene Glycol-Free Melphalan versus PG-Melphalan as Conditioning for Autologous Hematopoietic Cell Transplantation for Myeloma.Monahan K, Kleman A, Thapa B, Szabo A, D'Souza A, Dhakal B, Jerkins JH, Pasquini MC, Hamadani M, Hari PN, et al. Biol Blood Marrow Transplant. 2020 Dec; 26(12):2229-2236. Epub 2020 Sep 11.
- Review Cyclophosphamide.[LiverTox: Clinical and Researc...]Review Cyclophosphamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- A Phase IIb, Multicenter, Open-Label, Safety, and Efficacy Study of High-Dose, Propylene Glycol-Free Melphalan Hydrochloride for Injection (EVOMELA) for Myeloablative Conditioning in Multiple Myeloma Patients Undergoing Autologous Transplantation.[Biol Blood Marrow Transplant. ...]A Phase IIb, Multicenter, Open-Label, Safety, and Efficacy Study of High-Dose, Propylene Glycol-Free Melphalan Hydrochloride for Injection (EVOMELA) for Myeloablative Conditioning in Multiple Myeloma Patients Undergoing Autologous Transplantation.Hari P, Aljitawi OS, Arce-Lara C, Nath R, Callander N, Bhat G, Allen LF, Stockerl-Goldstein K. Biol Blood Marrow Transplant. 2015 Dec; 21(12):2100-2105. Epub 2015 Aug 29.
- Melphalan - LiverToxMelphalan - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...