NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mefenamic acid is a nonsteroidal antiinflammatory drug (NSAID) used largely for acute treatment of pain. Mefanamic acid has been linked to rare instances of clinically apparent, acute liver injury.
Background
Mefenamic (mef" e nam' ik) acid belongs to the anthranilic acid derivative class of NSAIDs (fenamates). Like other NSAIDs, mefenamic acid is a cyclo-oxygenase (Cox-1 and -2) inhibitor and blocks the production of intracellular prostaglandins that are important in pain and inflammatory pathways. Mefenamic acid has analgesic as well as antipyretic and antiinflammatory activities, but is used largely for treatment of pain. Mefenamic acid was approved in the United States in 1967, but is not a commonly used agent. Mefenamic acid is indicated for the treatment of mild-to-moderate acute pain or dysmenorrhea. It is available by prescription only in capsules of 250 and 500 mg in generic forms and under the brand name Ponstel. The recommended dose is 250 to 500 mg 3 to 4 times daily for periods of less than 7 days. Like most NSAIDs, mefenamic acid is generally well tolerated, but side effects can include headache, dizziness, somnolence, nausea, diarrhea, abdominal discomfort, heartburn, peripheral edema and hypersensitivity reactions.
Hepatotoxicity
In prospective studies less than 5% of patients taking mefenamic acid experienced transient serum aminotransferase elevations. The abnormalities usually resolved even while continuing the drug and without dose modification. Marked aminotransferase elevations (>3 fold elevated) occurred in <1% of patients. There have been isolated, rare cases of mefenamic acid induced liver injury published, but mefenamic acid is not mentioned as an etiologic agent in large case series on drug induced liver injury or acute liver failure. In most published cases, the liver injury arose as a part of a severe hypersensitivity reaction to mefenamic acid, such as Stevens Johnson syndrome or anaphylaxis. The latency to onset was short (less than one month) and the pattern injury variable from cholestatic to hepatocellular. The paucity of cases makes it difficult to characterize a typical clinical pattern of injury.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of mefenamic acid hepatotoxicity is not known, but is likely to be idiosyncratic hypersensitivity. The absence of reports of liver injury from mefenamic acid may be due, in part, to the infrequency of its use and limited duration of treatment.
Outcome and Management
The asymptomatic elevations in serum aminotransferase levels are usually self-limited and resolve even with continuing mefenamic acid. Severe cases of mefenamic acid hepatotoxicity, but not fatal cases or cases leading to chronic liver injury, have been described. Cross reactivity to hepatic injury among various classes of NSAIDs has not been well characterized; however, after clinically apparent injury attributable to mefenamic acid, it would be prudent to avoid other anthranilic acid derivatives (fenamates generally not available in the United States, such as tolfenamic acid and flufenamic acid), and patients who are switched to other NSAIDs should be monitored carefully.
Drug Class: Nonsteroidal Antiinflammatory Drugs
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mefenamic Acid – Ponstel®
DRUG CLASS
Nonsteroidal Antiinflammatory Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
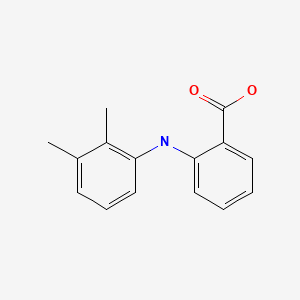
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mefenamic Acid | 61-68-7 | C15-H15-N-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 January 2020
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. The NSAIDS. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-41.(Review of hepatotoxicity of NSAIDs published in 1999: mentions that minor aminotransferase elevations occur in less than 5% of mefenamic acid recipients and was incriminated in an instance of “severe, but nonfatal, bridging necrosis”: Imoto).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd. Amsterdam: Elsevier, 2013, pp. 369-401.(Review of hepatotoxicity of NSAIDs mentions that mefenamic acid has been associated with only one published case of severe, but non-fatal hepatic necrosis: Imoto).
- Grosser T, Smyth E, FitzGerald GA. Anti-inflammatory, antipyretic, and analgesic agents; pharmacotherapy of gout. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 987-89. (Textbook of pharmacology and therapeutics).
- Imoto S, Matsumoto H, Fujii M. Drug-related hepatitis. Ann Intern Med 1979; 91: 129. 464433. [PubMed: 464433](Letter to editor stating that 35 patients with drug induced liver disease were seen over 6 years at a single Japanese center; 9 had severe bridging necrosis on liver biopsy; one case being attributed to mefenamic acid; but no details given of latency, test results, clinical features, course or competing diagnoses).
- Zimmerman HJ. Update of hepatotoxicity due to classes of drugs in common clinical use: non-steroid drugs, anti-inflammatory drugs, antibiotics, antihypertensives, and cardiac and psychotropic agents. Semin Liver Dis 1990; 10: 322-8. 2281340. [PubMed: 2281340](Extensive and excellent review article on liver injury due to NSAIDs; mefenamic acid is mentioned as being associated with a single case report of hepatocellular injury [Imoto]).
- Chan JCN, Lai FM, Critchley JAJH. A case of Stevens-Johnson syndrome, cholestatic hepatitis and haemolytic anaemia associated with use of mefenamic acid. Drug Saf 1991; 6: 230-4. 2064763. [PubMed: 2064763](44 year old woman developed rash after 2 weeks of mefenamic acid [with acetaminophen and furosemide] presenting with Stevens-Johnson syndrome, hemolytic anemia and bilirubin 18.0 mg/dL, ALT 427 U/L, Alk P 1220 U/L and renal failure progressing to multiorgan failure and death in 4 days, autopsy showed intrahepatic cholestasis and acute tubular necrosis).
- Schwartz D, Gremmel F, Kurz R, Tragl KH, Gellner B, Pausch V. Case report: acute renal failure, thrombocytopenia and nonhemolytic icterus probably caused by mefenamic acid(Parkemed)-dependent antibodies. Beitr Infusionsther 1992; 30: 413-5. 1284748. [PubMed: 1284748](65 year old man developed acute renal failure and thrombocytopenia shortly after a 3 day course of mefenamic acid followed by jaundice [bilirubin 20.7 mg/dL] which was attributed to "an isolated defect in glucuronization"; patient recovered and no other details given).
- de Mello NR, Baracat EC, Tomaz G, Bedone AJ, Camargos A, Barbosa IC, de Souza RN, et al. Double-blind study to evaluate efficacy and safety of meloxicam 7.5 mg and 15 mg versus mefenamic acid 1500 mg in the treatment of primary dysmenorrhea. Acta Obstet Gynecol Scand 2004; 83: 667-73. 15225193. [PubMed: 15225193](Controlled trial of 2 doses of meloxicam vs mefenamic acid for 3 to 5 days in 337 women with dysmenorrhea, aminotransferase elevations occurred in 2 of 227 meloxicam-, but in none of 110 mefenamic acid treated subjects).
- Somchit N, Sanat F, Gan EH, Shahrin IA, Zuraini A. Liver injury induced by the non-steroidal anti-inflammatory drug mefenamic acid. Singapore Med J 2004; 45: 530-2. 15510325. [PubMed: 15510325](Study in mice: dose dependent hepatotoxicity found with single intraperitoneally injection of 100-200 mg/kg as well as 14 day course at 50-100 mg/kg of mefenamic acid).
- Rostom A, Goldkind L, Laine L. Nonsteroidal anti-inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin Gastroenterol Hepatol 2005; 3: 489-98. 15880319. [PubMed: 15880319](Review of randomized clinical trial of NSAIDS for frequency of adverse events; ALT >3 fold ULN in 0.43% of ibuprofen, 0.43% naproxen, 0.42% celecoxib, 1.8% rofecoxib, 3.55% diclofenac and 0.29% of placebo recipients, rare liver related severe adverse events or deaths with any; no mention of mefenamic acid).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. 16165719. [PubMed: 16165719](Survey of all cases of drug induced liver injury with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002: among 103 cases, 3 attributed to naproxen, but none to mefenamic acid).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Del Pozo JG, Requejo AA, Arias LM, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol 2006; 20:391-5. 16867024. [PubMed: 16867024](Analysis of reports of liver injury from NSAIDs from France and Spain from 1982-2001; mefenamic acid was not listed among more than 29,000 liver adverse event reports).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. 18955056. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]; none for mefenamic acid).
- Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol 2010; 16: 5651-61. 21128314. [PMC free article: PMC2997980] [PubMed: 21128314](Review of estimated frequency of drug induced liver injury due to NSAIDs from large published epidemiological studies; no mention of mefenamic acid).
- Couto M, Duarte C, Geraldes L, Inês L, Malcata A. Anaphylaxis to mefenamic acid in a patient with new onset of systemic lupus erythematosus. Allergol Immunopathol (Madr) 2010; 38: 224-6. 20153574. [PubMed: 20153574](32 year old woman with lupus developed rash, followed by angioedema and anaphylactic shock a week after starting mefenamic acid for suspected lupus with accompanying hemolytic anemia, thrombocytopenia and liver test abnormalities [bilirubin 0.8 mg/dL, ALT 219 U/L, prothrombin time 22 sec], which rapidly improved with medical support and high dose corticosteroid therapy).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. 20949552. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 7 were due to NSAIDs, including 4 to bromfenac, 2 to diclofenac and 1 to etodolac, but none to mefenamic acid).
- Lapeyre-Mestre M, Grolleau S, Montastruc JL; Adsociation Française des Centres Régionaux de Pharmacovigilance (CRPV). Adverse drug reactions associated with the use of NSAIDs: a case/noncase analysis of spontaneous reports from the French pharmacovigilance database 2002-2006. Fundam Clin Pharmacol 2013; 27: 223-30. 21929527. [PubMed: 21929527](Analysis of 42,389 spontaneous serious adverse event reports to the French Pharmacovigilance database on 8 NSAIDs between 2002 and 2006; liver adverse events were most frequent with nimesulide [0.15 per million daily doses] compared to diclofenac [0.09], ketoprofen [0.09] piroxicam [0.06], naproxen [0.04], meloxicam [0.03], and tenoxicam [0.03]; mefenamic acid not discussed).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. 23419359. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 6 attributed to diclofenac [ranking 2nd], but none for mefenamic acid or other NSAIDs).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. 24552865. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996-2012 identified 176 cases, the most common class of implicated agents being NSAIDS [n=62, 32%], but specific agents were nimesulide [n=53], piroxicam [5], diclofenac [2], gold salts [1], and naproxen [1]; mefenamic acid was not listed]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. 25754159. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were attributed to NSAIDs, but none to mefenamic acid [Schmeltzer 2016]).
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW; Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int 2016; 36: 603-9. 26601797. [PMC free article: PMC5035108] [PubMed: 26601797](Among 1221 cases of drug induced liver injury enrolled in a prospective, US database between 2004 and 2014, 30 cases [2.5%] were attributed to NSAIDs, including 16 to diclofenac, 3 celecoxib, 3 meloxicam, 2 etodolac, 2 oxaprozin, 2 ibuprofen, 1 sulindac, and 1 valdecoxib, but none to mefenamic acid).
- Donati M, Conforti A, Lenti MC, Capuano A, Bortolami O, Motola D, Moretti U, et al.; DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol 2016; 82: 238-48. 26991794. [PMC free article: PMC4917796] [PubMed: 26991794](Among 179 cases of acute liver injury and 1770 controls admitted to 9 Italian hospitals between 2010 and 2014, NSAIDs used more frequently in cases compared to controls included nimesulide [17% vs 10%: odds ratio 1.88] and ibuprofen [14% vs 10%: odds ratio 1.59], and risk was higher in those taking higher doses; mefenamic acid is not listed).
- Sriuttha P, Sirichanchuen B, Permsuwan U. Hepatotoxicity of Nonsteroidal anti-inflammatory drugs: a systematic review of randomized controlled trials. Int J Hepatol 2018; 2018: 5253623. 29568654. [PMC free article: PMC5820561] [PubMed: 29568654](Systematic review of published randomized controlled trials for evidence of hepatotoxicity, found 18 studies that fit the stringent criteria, but none of them dealt with mefenamic acid).
- Ben-Nasr H, Ksouda K, Harrabi B, Hammami ST, Zeghal K, Affes H. Pediatric liver failure following mefenamic acid associated to herbal auto-medication: A case report. Therapie 2019; 74: 677-80. 31635831. [PubMed: 31635831](4 year old boy developed severe acute hepatocellular liver injury [ALT 1920 U/L, GGT 283 U/L, bilirubin 6.4 mg/dL, INR 1.52] 2 weeks after starting a mefenamic acid based cough medication and while intermittently receiving a home brewed herbal tea prepared from nutmeg [Mirystica fragrans], a flower [Hibiscus saabdariffa] and mint [Mentha aquatic], recovering within 3 weeks of presentation).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ketoprofen.[LiverTox: Clinical and Researc...]Review Ketoprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fenoprofen.[LiverTox: Clinical and Researc...]Review Fenoprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Flurbiprofen.[LiverTox: Clinical and Researc...]Review Flurbiprofen.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID‑19: A randomized double‑blind placebo‑controlled trial.[Int J Mol Med. 2022]Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID‑19: A randomized double‑blind placebo‑controlled trial.Guzman-Esquivel J, Galvan-Salazar HR, Guzman-Solorzano HP, Cuevas-Velazquez AC, Guzman-Solorzano JA, Mokay-Ramirez KA, Paz-Michel BA, Murillo-Zamora E, Delgado-Enciso J, Melnikov V, et al. Int J Mol Med. 2022 Mar; 49(3). Epub 2022 Jan 14.
- Review Nimesulide.[LiverTox: Clinical and Researc...]Review Nimesulide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Mefenamic Acid - LiverToxMefenamic Acid - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...