NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lumateperone is a second generation (atypical) antipsychotic agent that is used in the treatment of schizophrenia. Lumateperone is associated with a low rate of serum aminotransferase elevations during therapy, but has not been linked to instances of clinically apparent acute liver injury.
Background
Lumateperone (loo” ma te’ per one) is a second generation antipsychotic agent which appears to act as a serotonin (5-HT)-2A receptor and dopamine type 2 (D2) antagonist and is similar in structure and mechanism of action to paliperidone. It has low affinity for histamine-1 and alpha adrenergic-1 receptors and also is a partial serotonin reuptake inhibitor. Several randomized controlled trials have shown that oral lumateperone improves symptoms of schizophrenia and is comparable in effect to risperidone, but may be better tolerated. Lumateperone was approved for use in the United States in 2019 as treatment of adults with schizophrenia. Indications were subsequently extended to include depressive episodes in patients with bipolar I or II disorders including use as an adjunctive therapy with lithium or valproate. Lumateperone is available as capsules of 10.5, 21 and 42 mg under the brand name Caplyta. The recommended dosage in adults is 42 mg once daily. Common side effects include somnolence, fatigue, nausea, dizziness, dry mouth, nasal congestion, anxiety, restlessness and weight gain. Potential but rare severe adverse reactions (mentioned in most antipsychotic and antidepressant product labels) include excess mortality and cerebral vascular accidents in elderly patients with dementia, suicidal thoughts and behaviors, tardive dyskinesia, major neurologic events, neuroleptic malignant syndrome, orthostatic hypotension, seizures and neutropenia.
Hepatotoxicity
In preregistration controlled trials, ALT elevations arose in 2% of patients receiving lumateperone compared to less than 1% of placebo controls. The elevations, however, were usually mild, transient and typically resolved without dose modification or drug discontinuation. In preregistration trials, there were no instances of severe hepatic adverse events, discontinuations because of liver related events or episodes of clinically apparent liver injury with jaundice. Since its approval and more widescale use, there have been no published reports of liver injury with symptoms or jaundice attributed to lumateperone therapy, but clinical experience with its use has been limited.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which lumateperone might cause serum ALT elevations or liver injury is not known. Lumateperone is metabolized in the liver by multiple enzymes include CYP 3A4, 2C8 and 1A2 and drug-drug interactions may occur with agents that are strong inhibitors or inducers of hepatic microsomal enzymes.
Outcome and Management
The serum aminotransferase elevations that occur on lumateperone therapy are usually self-limited and often do not require dose modification or discontinuation. No instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been attributed to lumateperone. Cross sensitivity to liver related or other hypersensitivity reactions between lumateperone and structurally related antipsychotic agents (such as paliperidone, iloperidone, lurasidone, risperidone and ziprasidone) have not been demonstrated, but may well occur.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lumateperone – Caplyta®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
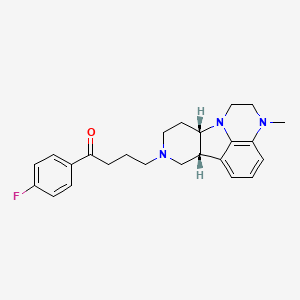
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lumateperone | 313368-91-1 | C24-H28-F-N3-O |
|
| Paliperidone | 144598-75-4 | C23-H27-F-N4-O3 |

|
ANNOTATED BIBLIOGRAPHY
References updated: June 6, 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/209500Orig1s000MultidisciplineR.pdf. (FDA website with product labels and the multidispline review of lumateperone that supported its approval for use in schizophrenia mentions ALT elevations arose in 2% of patients taking lumateperone vs 1% of controls and that 6 of 595 patients in one study developed ALT elevations above 3 times ULN during therapy, but all were less than 5 times ULN and most were self-limited and none were accompanied by jaundice). - Correll CU, Davis RE, Weingart M, Saillard J, O'Gorman C, Kane JM, Lieberman JA, et al. Efficacy and safety of lumateperone for treatment of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(4):349–358. [PMC free article: PMC6990963] [PubMed: 31913424](Among 450 adults with an acute exacerbation of schizophrenia treated with lumateperone [28 or 42 mg] or placebo once daily for 28 days, symptomatic response rates were higher with lumateperone [36% and 37% than placebo 26%] as were adverse event rates [57% and 65% vs 50%] including somnolence, sedation, fatigue and constipation; no mention of ALT elevations or hepatotoxicity).
- Lumateperone (Caplyta) for schizophrenia. Med Lett Drugs Ther. 2020;62(1603):113–116. [PubMed: 32728011](Concise review of the mechanism of action, clinical efficacy, safety and cost of lumateperone shortly after its approval for use in the US mentions common side effects of somnolence, nausea, dry mouth and elevations in serum aminotransferases).
- Greenwood J, Acharya RB, Marcellus V, Rey JA. Lumateperone: a novel antipsychotic for schizophrenia. Ann Pharmacother. 2021;55(1):98–104. [PubMed: 32590907](Review of the mechanism of action, clinical efficacy and safety of lumateperone mentions that it has high affinity for the serotonin 2A receptor, moderate affinity for dopamine 1 receptor, and may also act as a serotonin-reupdate inhibitor; in two short term clinical trials, lumateperone improved both negative and positive symptoms of schizophrenia, but long term results with comparisons to other second generation antipsychotics have not been reported).
- Correll CU, Vanover KE, Davis RE, Chen R, Satlin A, Mates S. Safety and tolerability of lumateperone 42 mg: an open-label antipsychotic switch study in outpatients with stable schizophrenia. Schizophr Res. 2021;228:198–205. [PubMed: 33453691](Among 301 adults with stable schizophrenia who were switched from another atypical antipsychotic to lumateperone for 6 weeks, symptom scores were stable while 31% developed a drug related adverse event which were mostly mild-to-moderate somnolence [7%], headache [5%] and dry mouth [5%], and among the severe adverse events [3%], none were liver related; no mention of ALT elevations).
- D'Souza I, Durgam S, Satlin A, Davis RE, Kozauer SG, Chen R, Mates S, et al. Lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. CNS Spectrums. 2021;26:150. Not in PubMed. [PubMed: 36779257](Abstract only: among 377 adults with bipolar disorders experiencing a manic episode treated with lumateperone [42 mg] vs placebo daily for 6 weeks, remission rates were 51% vs 37% at day 43, and therapy was “generally well tolerated”; no information on ALT levels or hepatotoxicity).
- Kane JM, Durgam S, Satlin A, Vanover KE, Chen R, Davis R, Mates S. Safety and tolerability of lumateperone for the treatment of schizophrenia: a pooled analysis of late-phase placebo- and active-controlled clinical trials. Int Clin Psychopharmacol. 2021;36:244–250. [PMC free article: PMC8322041] [PubMed: 34054112](Pooled results from 3 randomized, double-blind controlled trials of lumateperone [n=406] vs risperidone [n=255] and placebo [n=411] for 4 or 6 weeks, overall adverse event rates were 66%, 69% and 55% and there were no deaths and no liver related discontinuations or serious adverse events, and while there were minor average increases in ALT, AST and Alk P on lumateperone therapy, there were no instances of liver injury with jaundice).
- Calabrese JR, Durgam S, Satlin A, Vanover KE, Davis RE, Chen R, Kozauer SG, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178:1098–1106. [PubMed: 34551584](Among 377 adults with bipolar disorders experiencing a major depressive episode enrolled in a randomized controlled trial for 6 weeks, symptom scores improved more with lumateperone [42 mg daily] than placebo and adverse events arose in 55% vs 50%, but there were no serious hepatic adverse events; no mention of ALT elevations or hepatotoxicity).
- Lumateperone (Caplyta) for bipolar depression. Med Lett Drugs Ther. 2022;64(1656):126–128. [PubMed: 35921078](Concise review of the mechanism of action, clinical efficacy, safety, and cost of lumateperone shortly after its approval in the US for treatment of bipolar depression; does not mention ALT elevations or hepatotoxicity).
- Suppes T, Durgam S, Kozauer SG, Chen R, Lakkis HD, Davis RE, Satlin A, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: Results from a randomized placebo-controlled clinical trial. Bipolar Disord. 2023 Feb 13; In Press. [PubMed: 36779257](Among 529 adults with bipolar I and II disorder on valproate or lithium to which was added lumateperone [28 or 42 mg] or placebo once daily for 6 weeks, depression symptom scores improved more with lumateperone than placebo [-16.2 and -16.9 vs -14.5], while total adverse event rates were similar and side effects more frequent with lumateperone included somnolence, dizziness and nausea; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Iloperidone.[LiverTox: Clinical and Researc...]Review Iloperidone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Lurasidone.[LiverTox: Clinical and Researc...]Review Lurasidone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Paliperidone.[LiverTox: Clinical and Researc...]Review Paliperidone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Asenapine.[LiverTox: Clinical and Researc...]Review Asenapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Safety and tolerability of lumateperone 42 mg: An open-label antipsychotic switch study in outpatients with stable schizophrenia.[Schizophr Res. 2021]Safety and tolerability of lumateperone 42 mg: An open-label antipsychotic switch study in outpatients with stable schizophrenia.Correll CU, Vanover KE, Davis RE, Chen R, Satlin A, Mates S. Schizophr Res. 2021 Feb; 228:198-205. Epub 2021 Jan 13.
- Lumateperone - LiverToxLumateperone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...