NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lorazepam is an orally available benzodiazepine used widely in the therapy of anxiety and as a liquid solution as therapy of status epilepticus and for preoperative sedation. As with most benzodiazepines, lorazepam has not been associated with serum aminotransferase or alkaline phosphatase elevations during therapy, and a single case of clinically apparent liver injury from lorazepam has been reported in the literature despite its wide scale use for several decades.
Background
Lorazepam (lor az' e pam) is a benzodiazepine that is widely used orally in the therapy of anxiety. Lorazepam is also used in parenteral form for therapy of status epilepticus and in preoperative sedation and management of nausea and vomiting. The antianxiety (anxiolytic) and soporific activity of the benzodiazepines is mediated by their ability to enhance gamma-aminobutyric acid (GABA) mediated inhibition of synaptic transmission through binding to the GABA A receptor. Lorazepam was approved in the United States in 1977 as therapy for anxiety disorders, and currently more than 10 million prescriptions are filled yearly. Indications include management of anxiety disorders and short term relief of symptoms of anxiety. Lorazepam is available in tablets of 0.5, 1 and 2 mg in several generic forms and under the brand name Ativan. Parenteral formulations of lorazepam (2 and 4 mg per mL) are available for use in status epilepticus and for preoperative sedation and control of nausea and vomiting after cancer chemotherapy. The recommended oral dose for adults is 0.5 to 1 mg two to three times daily, increasing as needed to a maximum dose of 10 mg daily in divided doses. The most common side effects of lorazepam are dose related and include drowsiness, lethargy, ataxia, dysarthria and dizziness. Tolerance develops to these side effects, but tolerance may also develop to the effects on anxiety and insomnia. Lorazepam like all oral benzodiazepines has a boxed warning in its product label stressing (1) the risks of severe sedation and potentially fatal respiratory depression when combined with opiates, (2) with prolonged use, the risks of abuse, misuse, and addiction which can lead to overdose and death, and (3) with continued use, the risks of dependence and severe, potentially life-threatening withdrawal symptoms if discontinued suddenly.
Hepatotoxicity
Lorazepam, as with other benzodiazepines, is rarely associated with serum ALT or alkaline phosphatase elevations during therapy, and considering its widescale use, clinically apparent liver injury from lorazepam is extremely rare. There has been only a single case report of symptomatic, acute liver injury from lorazepam. The latency to onset was 9 months and the clinical pattern that of a self-limited cholestatic hepatitis that resolved within 2 months of stopping. Similar isolated single cases of clinically apparent hepatitis have been reported with other benzodiazepines including alprazolam, chlordiazepoxide, clonazepam, diazepam, flurazepam and triazolam. The clinical pattern of acute liver injury from benzodiazepines is typically cholestatic and mild-to-moderate in severity with a latency of 1 to 6 months. Fever and rash are not common nor is autoantibody formation.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Lorazepam is metabolized by the liver to inactive metabolites and is considered the benzodiazepine best tolerated by patients with advanced liver disease. Liver injury from benzodiazepines is probably due to the toxic effects of a rarely produced intermediate metabolite.
Outcome and Management
The case reports of hepatic injury due to benzodiazepines were followed by prompt and complete recovery upon stopping the medication, without evidence of residual or chronic injury. No cases of acute liver failure or chronic liver injury due to lorazepam have been described. There is no information about cross reactivity with other benzodiazepines, but some degree of cross sensitivity may occur.
Drug Class: Sedatives and Hypnotics, Benzodiazepines, Antianxiety Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lorazepam – Generic, Ativan®
DRUG CLASS
Benzodiazepines
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
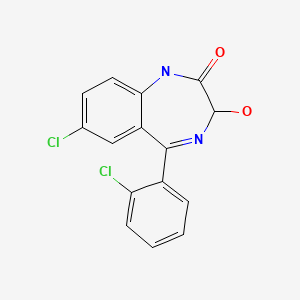
| Lorazepam | 846-49-1 | C15-H10-Cl2-N2-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 22 June 2023
- Zimmerman HJ. Benzodiazepines. Psychotropic and anticonvulsant agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 491-3.(Expert review of benzodiazepines and liver injury published in 1999; mentions rare instances of cholestatic hepatitis have been reported due to alprazolam, chlordiazepoxide, diazepam, flurazepam, and triazolam, and hepatocellular injury with clorazepate and clotiazepam, but no reports of hepatic injury with lorazepam, oxazepam, or temazepam).
- Larrey D, Ripault MP. Benzodiazepines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsever, 2013, pp. 455.(Review of liver injury due to psychotropic drugs; rare instances of acute liver injury [usually cholestatic] have been reported with alprazolam, clonazepam, chlordiazepoxide, diazepam, flurazepam, triazolam, and bentazepam).
- Mihic SJ, Mayfield J, Harris RA. Hypnotics and sedatives. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 339-53.(Textbook of pharmacology and therapeutics).
- Davion T, Capron-Chivrac D, Andrejak M, Capron JP. Gastroenterol Clin Biol. 1985;9:117–26. [Hepatitis due to antiepileptic agents] [PubMed: 3920108](Review of hepatotoxicity of anticonvulsants; among benzodiazepines, cases of cholestatic hepatitis have been linked to chlordiazepoxide and diazepam, but liver injury from this class of drugs is exceptionally rare).
- Lewis JH, Zimmerman HJ. Drug- and chemical-induced cholestasis. Clin Liver Dis. 1999;3:433–64. vii. Erratum in: Clin Liver Dis 1999; 3: 917. [PubMed: 11291233](Review of drug induced cholestatic syndromes, listing many causes including chlordiazepoxide and flurazepam; “Benzodiazepines may cause cholestatic injury, although this is rare”).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 20 were attributed to benzodiazepines including 5 for clorazepate, 5 alprazolam, 6 lorazepam and 4 diazepam, but compared to controls, the relative risk of injury was increased only for clorazepate [8.3 and frequency 3.4 per 100,000 person-year exposures]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to a benzodiazepine).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of hepatotoxicity of all anticonvulsants focusing upon phenytoin, valproate, carbamazepine; “Furthermore, hepatoxicity has not been convincingly shown to be associated with the use of benzodiazepines”).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to a benzodiazepine).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to a benzodiazepine, despite the fact that millions of prescriptions are filled yearly).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature on drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to a benzodiazepine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, no cases were attributed to lorazepam or any other benzodiazepine).
- Arai T, Kogi K, Honda Y, Suzuki T, Kawai K, Okamoto M, Fujioka T, et al. Lorazepam as a cause of drug-induced liver injury. Case Rep Gastroenterol. 2018;12:546–550. [PMC free article: PMC6167696] [PubMed: 30283291](51 year old Japanese man with depression developed jaundice 9 months after starting lorazepam [bilirubin 26.8 mg/dL, ALT 99 U/L, Alk P 974 U/L, normal <335 U/L], with resolution within 2 months of stopping but was taking several other psychotropic agents which were also stopped).
- Drugs for anxiety disorders. Med Lett Drugs Ther. 2019;61:121–6. [PubMed: 31386647](Concise review of drugs for anxiety including SSRIs, SNRIs and benzodiazepines including mechanism of action, clinical efficacy, safety, and costs; does not mention ALT elevations or hepatotoxicity).
- Drugs for chronic insomnia. Med Lett Drugs Ther. 2023;65:1–6. [PubMed: 36630579](Concise review of drugs for chronic insomnia mentions that tolerance and dependence can occur with use of benzodiazepines and their use should be discouraged, and that benzodiazepines are CNS suppressants and can impair next day performance including driving and cause complex behavior disorders, retrograde amnesia, dependence, tolerance, abuse and rebound insomnia; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Patterns of benzodiazepine underdosing in the Established Status Epilepticus Treatment Trial.[Epilepsia. 2021]Patterns of benzodiazepine underdosing in the Established Status Epilepticus Treatment Trial.Sathe AG, Underwood E, Coles LD, Elm JJ, Silbergleit R, Chamberlain JM, Kapur J, Cock HR, Fountain NB, Shinnar S, et al. Epilepsia. 2021 Mar; 62(3):795-806. Epub 2021 Feb 10.
- Review Alprazolam.[LiverTox: Clinical and Researc...]Review Alprazolam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Oxazepam.[LiverTox: Clinical and Researc...]Review Oxazepam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Chlordiazepoxide.[LiverTox: Clinical and Researc...]Review Chlordiazepoxide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Flurazepam.[LiverTox: Clinical and Researc...]Review Flurazepam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Lorazepam - LiverToxLorazepam - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...