NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lomitapide is a cholesterol lowering agent that acts by inhibition of the microsomal triglyceride transfer protein and is used to treat the severe lipid abnormalities of familial hypercholesterolemia. Lomitapide is associated with mild, asymptomatic and self-limited serum aminotransferase elevations during therapy that are usually accompanied by an increase in hepatic fat. Long term therapy with lomitapide has been linked to development of steatohepatitis and hepatic fibrosis.
Background
Lomitapide (loe mi' ta pide) is a potent, orally available inhibitor of the hepatic microsomal triglyceride transfer protein (MTTP) and is used to treat severe forms of familial hypercholesterolemia. MTTP is responsible for transferring triglyceride to apolipoprotein B in the liver which is necessary for the assembly of very low density lipoproteins, the precursors of low density lipoproteins (LDL). Inhibition of apolipoprotein B assembly leads to a marked decrease in circulating LDL cholesterol and triglycerides. Lomitapide is typically used as an adjunct to a low-fat diet and other cholesterol-lowering treatments including statins. Lomitapide was approved for use in the United States in 2012, but its indications were limited to patients with homozygous familial hypercholesterolemia. Use of lomitapide can be associated with serum aminotransferase elevations and increase in hepatic fat. Because of the risk of liver injury, lomitapide is available only as a part of a "Risk Evaluation and Mitigation Strategy" [REMS] that requires regular monitoring of liver tests. Lomitapide is available in capsules of 5, 10, 20, 30, 40 and 60 mg under the trade name Juxtapid. The recommended dose is 5 mg daily initially, with subsequent increases based upon tolerance and effectiveness to a maximum of 60 mg daily. Side effects are not uncommon and include diarrhea, nausea, dyspepsia and abdominal pain that are ameliorated and partially prevented by strict adherence to a low fat diet. Lomitapide can also cause marked drug-drug interactions and increase toxicities of other medications (statins, warfarin, antibiotics). Chronic use of lomitapide can result in fat-soluble vitamin malabsorption and deficiencies.
Hepatotoxicity
Lomitapide is associated with a moderately high rate of serum aminotransferase elevations during therapy, levels above 3 times the upper limit of normal (ULN) occurring in 34% of patients. Aminotransferase elevations above 10 times ULN have also been reported which can necessitate drug discontinuation. Despite the frequency of ALT elevations, however, increases in serum bilirubin and alkaline phosphatase levels are rare and there have been no reports of clinically apparent acute liver injury with jaundice. Chronic therapy with lomitapide can be associated with fluctuations in serum aminotransferase levels and accumulation of liver fat. In some instances, the increase in liver fat is from baseline levels of <2% to as high as 10% to 40%. At least one instance of steatohepatitis and progressive hepatic fibrosis has been reported in a patient receiving lomitapide long term. The hepatic steatosis is reversed upon stopping lomitapide and generally does not progressively accumulate. The reason why some patients develop liver test abnormalities and accumulate significant amounts of liver fat on lomitapide therapy while others do not, is not clear. The effect is clearly dose related and serum enzyme elevations generally improve with dose modification. The frequency of liver test abnormalities and their association with steatohepatitis led to the requirement for a Risk Evaluation and Mitigation Strategy for lomitapide and it is only available for patients registered in a REMS program.
Likelihood score: C (probable cause of clinically significant liver injury).
Mechanism of Injury
The cause of hepatic injury from lomitapide appears to be a direct effect of its mechanism of action in inhibiting triglyceride transport out of hepatocytes, which leads to hepatocyte steatosis and, in some instances, liver injury. Lomitapide is also extensively metabolized in the liver, primarily via CYP 3A4 and is very sensitive to inhibitors of this microsomal enzyme. Thus, strong CYP 3A4 inhibitors can cause a marked increase in lomitapide levels. Furthermore, lomitapide can compete with other medications in CYP 3A4 metabolism and cause increases in their levels. Lomitapide is also an inhibitor of P-glycoprotein and can increase the absorption of its substrates.
Outcome and Management
The ALT elevations associated with lomitapide therapy are not uncommon and are often accompanied by increases in hepatic steatosis which may ultimately lead to steatohepatitis and significant chronic liver injury. The REMS management program calls for dose adjustment or drug discontinuation based upon the degree of serum aminotransferase elevations. Patients receiving lomitapide should also be advised to follow a low fat diet and take fat soluble vitamin supplements. Lomitapide can also cause drug-drug interactions and caution should be used in co-administration of lomitapide with other inhibitors or substrates of CYP 3A4, P-glycoprotein substrates and warfarin.
Drug Class: Antilipemic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lomitapide – Juxtapid®
DRUG CLASS
Antilipemic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
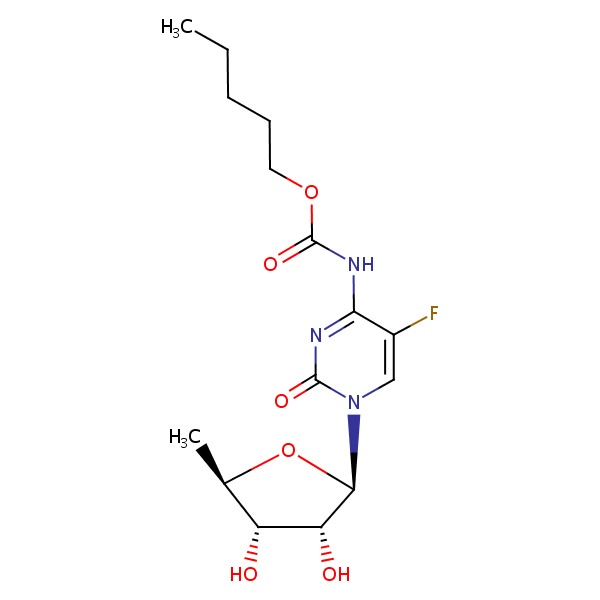
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lomitapide | 182431-12-5 | C39-H37-F6-N3-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 20 May 2019
- Zimmerman HJ. Drugs used in the treatment of hypercholesterolemia and hyperlipidemia. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 660-2.(Expert review of hepatotoxicity published in 1999, before the availability of lomitapide).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic medications. Lipid lowering agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of lipid lowering agents; lomitapide is not discussed).
- Gurgle HE, Blumenthal DK. Drug therapy for dyslipidemias. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 605-18.(Textbook of pharmacology and therapeutics).
- Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med 2007; 356: 148-56. [PubMed: 17215532](Among 6 patients with familial hypercholesterolemia treated with 4 doses of lomitapide for 4 weeks, LDL cholesterol levels decreased by 51% and triglycerides by 65% at the highest dose and ALT elevations arose in 4 patients, all of whom also had increases in hepatic fat by MRI [10-40%]).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 6 due to statins, but none to lomitapide).
- Two new drugs for homozygous familial hypercholesterolemia. Med Lett Drugs Ther 2013; 55 (1413): 25-7. [PubMed: 23545581](Concise review of the mechanism of action, efficacy, safety and costs of lomitapide shortly after its approval for use in the US; mentions that it can cause elevations in aminotransferase levels and increases in hepatic steatosis for which reason it is only available through a restricted access program).
- Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, et al.; Phase 3 HoFH Lomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 2013; 381 (9860): 40-6. [PMC free article: PMC4587657] [PubMed: 23122768](Among 28 patients with familial hypercholesterolemia treated with escalating doses of lomitapide [5 to 60 mg daily], LDL cholesterol decreased by 50%, but side effects were common, mostly gastrointestinal [>90%] and ALT elevations above 3 times ULN occurred in 10 [36%] and above 5 times ULN in 4 [14%], but none required drug discontinuation and none were associated with jaundice or symptoms, but were accompanied by increases in liver fat [from <2% to ~8%]).
- Raal FJ. Lomitapide for homozygous familial hypercholesterolaemia. Lancet 2013; 381 (9860): 7-8. [PubMed: 23122767](Editorial on familial hypercholesterolemia in response to article by Cuchel et al. [2013]).
- Davis KA, Miyares MA. Lomitapide: A novel agent for the treatment of homozygous familial hypercholesterolemia. Am J Health Syst Pharm 2014; 71: 1001-8. [PubMed: 24865757](Review of the mechanism of action, efficacy and side effects of lomitapide).
- Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014; 129: 1022-32. [PubMed: 24589695](Summary of mechanism of action of microsomal triglyceride transport protein [MTTP] inhibitors, their efficacy, adverse event rates, clinical usefulness and need for further research).
- Sacks FM, Stanesa M, Hegele RA. Progression to hepatitis and fibrosis secondary to lomitapide use--reply. JAMA Intern Med 2014; 174: 1522-3. [PubMed: 25178869](44 year old woman with severe hypertriglyceridemia and recurrent pancreatitis was treated with lomitapide for 13 years with excellent control of lipid levels, but with fluctuating ALT levels and development of steatohepatitis eventually with significant fibrosis).
- Miyares MA. Progression to hepatitis and fibrosis secondary to lomitapide use: selecting the next course of action. JAMA Intern Med 2014; 174: 1522. [PubMed: 25178868](Letter in response to article by Sacks [2014] questioning the use of lomitapide for 13 years in the face of signs of steatohepatitis).
- Cuchel M, Blom DJ, Averna MR. Clinical experience of lomitapide therapy in patients with homozygous familial hypercholesterolaemia. Atheroscler Suppl 2014; 15: 33-45. [PubMed: 25257075](Clinical review of efficacy and safety of lomitapide in 29 patients with familial hypercholesterolemia [Cuchel 2013] with in depth discussion of management of side effects including serum ALT elevations).
- Goulooze SC, Cohen AF, Rissmann R. Lomitapide. Br J Clin Pharmacol 2015; 80: 179-81. [PMC free article: PMC4541964] [PubMed: 25702706](Brief review of the mechanism of action and clinical use of lomitapide).
- Roeters van Lennep J, Averna M, Alonso R. Treating homozygous familial hypercholesterolemia in a real-world setting: Experiences with lomitapide. J Clin Lipidol 2015; 9: 607-17. [PubMed: 26228681](Details of 4 European patients with familial hypercholesterolemia treated in the "real-world" including one who developed recurrent serum ALT elevations [from 16 to 230 U/L and then from 48 to 132 U/L] and increased liver fat with modest doses of lomitapide [5-10 mg daily], ultimately requiring drug discontinuation).
- Raper A, Kolansky DM, Sachais BS, Meagher EA, Baer AL, Cuchel M. Long-term clinical results of microsomal triglyceride transfer protein inhibitor use in a patient with homozygous familial hypercholesterolemia. J Clin Lipidol 2015; 9: 107-12. [PMC free article: PMC4563802] [PubMed: 25670368](49 year old woman with familial hypercholesterolemia and cardiac complications was treated with lomitapide [40-60 mg daily] and had intermittent ALT elevations [peak ~10 times ULN, but without jaundice or symptoms], but responded to discontinuation and abnormalities did not recur on restarting).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 41 cases [5.4%] were attributed to lipid-lowering agents, including 31 due to statins, 5 to niacin and 5 to fibrates, but none to lomitapide).
- Lomitapide (Lojuxta). Use only in homozygous familial hypercholesterolaemia, with caution. Prescrire Int 2015; 24: 176-8. [PubMed: 26240881](Commentary on lomitapide stressing its potential for causing liver injury and need to limit its use to patients with homozygous familial hypercholesterolemia).
- Stefanutti C, Morozzi C, Di Giacomo S, Sovrano B, Mesce D, Grossi A. Management of homozygous familial hypercholesterolemia in real-world clinical practice: A report of 7 Italian patients treated in Rome with lomitapide and lipoprotein apheresis. J Clin Lipidol 2016; 10: 782-9. [PubMed: 27578108](Description of 7 patients with familial hypercholesterolemia treated with lomitapide, all on lipoprotein apheresis, in whom careful titration of dose was associated with lower rates of gastrointestinal side effects and no patient had to stop therapy because of ALT elevations).
- Sanna C, Stéphenne X, Revencu N, Smets F, Sassolas A, Di Filippo M, Descamps OS, et al. Homozygous familial hypercholesterolemia in childhood: Genotype-phenotype description, established therapies and perspectives. Atherosclerosis 2016; 247: 97-104. [PubMed: 26894473](Among 8 patients with familial hypercholesterolemia, all had LDL-cholesterol levels of 500 mg/dL and only modest improvements with use of statins and ezetimibe compared to marked improvements after liver transplantation).
- D'Erasmo L, Cefalù AB, Noto D, Giammanco A, Averna M, Pintus P, Medde P, et al. Efficacy of lomitapide in the treatment of familial homozygous hHypercholesterolemia: results of a real-world clinical experience in Italy. Adv Ther 2017; 34: 1200-10. [PubMed: 28432645](Among 15 Italian patients with familial hypercholesterolemia treated with lomitapide, LCL-cholesterol levels fell in all from a mean of 493 to a nadir of 132 mg/dL, and no patient had ALT elevations above 5 times ULN or required discontinuation for liver test abnormalities).
- Chacra APM, Ferrari MC, Rocha VZ, Santos RD. Case report: The efficiency and safety of lomitapide in a homozygous familial hypercholesterolemic child. J Clin Lipidol 2019 Mar 11. pii: [Epub ahead of print] [PubMed: 30948303](7.6 year old girl with familial hypercholesterolemia was treated with lomitapide and after 3.5 years LDL-cholesterol levels fell from 428 to 266 mg/dL and ALT and AST levels remained unchanged).
- Ben-Omran T, Masana L, Kolovou G, Ariceta G, Nóvoa FJ, Lund AM, Bogsrud MP, et al. Real-world outcomes with lomitapide use in paediatric patients with homozygous familial hypercholesterolaemia. Adv Ther 2019 May 17. [Epub ahead of print] [PMC free article: PMC6824397] [PubMed: 31102204](Among 11 children at 10 centers in 8 countries treated with lomitapide, LDL-cholesterol levels fell from a mean of 419 to a nadir of 177 mg/dL, and 3 had ALT elevations but were managed with dose adjustment without stopping therapy).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Long-term hepatic safety of lomitapide in homozygous familial hypercholesterolaemia.[Liver Int. 2023]Long-term hepatic safety of lomitapide in homozygous familial hypercholesterolaemia.Larrey D, D'Erasmo L, O'Brien S, Arca M, Italian Working Group on Lomitapide. Liver Int. 2023 Feb; 43(2):413-423. Epub 2022 Dec 30.
- Review Efficacy and Safety of Lomitapide in Hypercholesterolemia.[Am J Cardiovasc Drugs. 2017]Review Efficacy and Safety of Lomitapide in Hypercholesterolemia.Liu X, Men P, Wang Y, Zhai S, Zhao Z, Liu G. Am J Cardiovasc Drugs. 2017 Aug; 17(4):299-309.
- Microsomal triglyceride transfer protein inhibitor (lomitapide) efficacy in the treatment of patients with homozygous familial hypercholesterolaemia.[Eur J Prev Cardiol. 2020]Microsomal triglyceride transfer protein inhibitor (lomitapide) efficacy in the treatment of patients with homozygous familial hypercholesterolaemia.Kolovou G, Diakoumakou O, Kolovou V, Fountas E, Stratakis S, Zacharis E, Liberopoulos EN, Matsouka F, Tsoutsinos A, Mastorakou I, et al. Eur J Prev Cardiol. 2020 Jan; 27(2):157-165. Epub 2019 Aug 12.
- Microsomal triglyceride transfer protein inhibitor lomitapide-induced liver toxicity is ameliorated by Triiodothyronine treatment following improved bile homeostasis and β-oxidation.[Toxicol Appl Pharmacol. 2022]Microsomal triglyceride transfer protein inhibitor lomitapide-induced liver toxicity is ameliorated by Triiodothyronine treatment following improved bile homeostasis and β-oxidation.Patel V, Joharapurkar A, Kshirsagar S, Patel M, Patel H, Savsani H, Jain M. Toxicol Appl Pharmacol. 2022 Jan 1; 434:115825. Epub 2021 Dec 10.
- Review Lomitapide and Mipomersen-Inhibiting Microsomal Triglyceride Transfer Protein (MTP) and apoB100 Synthesis.[Curr Atheroscler Rep. 2019]Review Lomitapide and Mipomersen-Inhibiting Microsomal Triglyceride Transfer Protein (MTP) and apoB100 Synthesis.Blom DJ, Raal FJ, Santos RD, Marais AD. Curr Atheroscler Rep. 2019 Nov 19; 21(12):48. Epub 2019 Nov 19.
- Lomitapide - LiverToxLomitapide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...