NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lithium is a simple alkali metal, the salt of which acts as a mood stabilizing agent which has been extensively used for the treatment of mania for more than 50 years. Lithium has been associated with rare instances of mild serum aminotransferase elevations, but has not been convincingly linked to clinically apparent acute liver injury.
Background
Lithium (lith' ee um) is the lightest elemental metal and is normally found in low concentrations in human tissue. Lithium salts are highly water soluble and have been used medically for many years. While lithium has no psychotropic effects in normal individuals, it has potent mood stabilizing properties in patients with bipolar disorders, mania and recurrent depression. The mechanism of action of lithium is unknown, but is thought to be mediated by its replacement of sodium ions and disruption of membrane potentials in the central nervous system. It may also act by differential effects on neurotransmitter induced depolarization of membranes or interference with phosphatidylinositol pathways. Lithium was approved for use in bipolar illness in the United States in 1970 and it is still widely used for this indication. Lithium has also been used off-label in therapy of schizophrenia, alcohol dependence, attention deficit disorder and migraine headaches. Lithium is available as capsules or tablets of 150, 300, 450 and 600 mg in generic forms as well in several brand names including Carbolith, Duralith and Eskalith. A typical maintenance dose regimen is 600 to 900 mg daily. Lithium levels are generally monitored because of the narrow therapeutic window between toxicity and effectiveness aiming for levels between 0.6 and 1.2 mEq/L in chronic situations (higher in acute). Common side effects include metallic taste, nausea, tremor, polyuria, polydipsia and weight gain. Uncommon side effects include hypothyroidism. The major serious adverse event is severe lithium toxicity with can include polyuria, hyponatremia, renal disease and encephalopathy. Because of its narrow therapeutic window, lithium requires careful monitoring of drug levels.
Hepatotoxicity
Liver test abnormalities have been reported to occur in a small proportion of patients on long term therapy with lithium. These abnormalities are usually asymptomatic and transient, reversing even with continuation of medication. Instances of more marked elevations in serum aminotransferases have been reported in patients taking overdoses of lithium, but the other metabolic and systemic effects of lithium overdose generally overshadow hepatic adverse effects. Lithium has not been associated with instances of clinically apparent acute liver injury with jaundice.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which lithium causes serum aminotransferase elevations is not known. Some instances of serum aminotransferase elevations occurring on lithium therapy may be due to nonalcoholic fatty liver disease caused by weight gain that occurs in at least one-quarter of treated patients and ranges from 4.5 to 12 kg, generally during the first 1 to 2 years of therapy.
Outcome and Management
The serum aminotransferase elevations that occur on lithium therapy are usually self-limited and do not require dose modification or discontinuation of therapy. No instances of acute liver failure or chronic liver disease have been attributed to lithium.
Drug Class: Antipsychotic Agents, Drugs for Bipolar Disorders, Trace Elements and Metals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lithium – Generic, Carbolith®, Duralith®, Eskalith®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
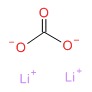
| Lithium Carbonate | 554-13-2 | C-H2-O3.2Li |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 04 June 2019
- Zimmerman HJ. Psychotropic and anticonvulsant agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-516.(Expert review of hepatotoxicity of psychiatric medications published in 1999; lithium is not discussed).
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- McKnelly WV Jr, Tupin JP, Dunn M. Lithium in hazardous circumstances with one case of lithium toxicity. Compr Psychiatry 1970; 11: 279-86. [PubMed: 5443145](4 cases of use of lithium in patients with other significant conditions; one patient with heart failure developed jaundice and hepatomegaly attributed to passive congestion rather than lithium).
- Warick LH. Lithium poisoning. Report of a case with neurologic, cardiac and hepatic sequelae. West J Med 1979; 130: 259-63. [PMC free article: PMC1238593] [PubMed: 425510](36 year old man on high doses of lithium for 10 days developed drowsiness, ataxia, nausea and vomiting with high lithium levels, ALT rising to 911 U/L, Alk P to 178 U/L, normal bilirubin; no follow up on resolution).
- Cohen LS, Cohen DE. Lithium-induced hyperbilirubinemia in an adolescent. J Clin Psychopharmacol 1991; 11: 274-5. [PubMed: 1918429](17 year old man developed bilirubin elevations after taking lithium for 12 days [rising from 1.4 at baseline to 3.3 mg/dL], while all other tests were normal; authors suspected Gilbert syndrome exacerbated by lithium).
- Kosson H, Chou JC-Y. Abnormal liver function tests associated with lithium treatment. J Clin Psychopharmacol 1992; 12: 216-7. [PubMed: 1629393](26 year old man developed mild increases in ALT levels [peak 105 U/L] after 2 months of lithium treatment which decreased on stopping and increased again on restarting on two occasions [from 11 to 56 U/L and 19 to 56 U/L]; no symptoms and no mention of bilirubin elevations or weight gain).
- Smith LA, Cornelius V, Warnock A, Tacchi MJ, Taylor D. Pharmacological interventions for acute bipolar mania: a systematic review of randomized placebo-controlled trials. Bipolar Disorders 2007; 9: 551-60. [PubMed: 17845269](Review of 13 short term controlled trials with 3089 patients, weight gain with olanzapine and risperidone, no mention of hepatic adverse events).
- Smith LA, Cornelius V, Warnock A, Bell A, Young AH. Effectiveness of mood stabilizers and antipsychotics in the maintenance phase of bipolar disorder: a systematic review of randomized controlled trials. Bipolar Disord 2007; 9: 394-412. [PubMed: 17547586](Review of 14 long term controlled trials of mood stabilizing agents including lithium with 2526 patients, did not evaluate adverse events).
- Torrent C, Amann B, Sanchez-Moreno J, Colom F, Feinares M, Comes M, Rosa AR, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand 2008; 118: 4-18. [PubMed: 18498432](Review of frequency of weight gain in patients treated for bipolar disorders, most weight gain occurred with clozapine and olanzapine, but some weight gain also with quetiapine, risperidone, lithium, valproate and gabapentin, but not with carbamazepine or lamotrigine).
- Young W. Review of lithium effects on brain and blood. Cell Transplant 2009; 18: 951-75. [PubMed: 19523343](Review of the mechanism of action of lithium, its clinical uses and toxicity; states that prolonged exposure to high doses of lithium [above 2 mM] can cause liver injury).
- McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: a systematic review and meta-analysis. Lancet 2012; 379 (9817): 721-8. [PubMed: 22265699](A systematic review of the literature on litium toxicity focusing upon renal thyroid and parathyroid function, hair and skin disorders, weight gain and teratogenicity; no mention of hepatic side effects).
- Drugs for psychiatric disorders. Treat Guidel Med Lett 2013; 11 (130): 53-64. [PubMed: 23715100](Concise review of the safety and efficacy of drugs for psychiatric disorders states that: "Lithium is generally the drug of choice for maintenace treatment of bipolar disorder"; no mention of hepatic adverse events).
- Drugs for bipolar disorder. Med Lett Drugs Ther 2016; 58 (1501): 103-6. [PubMed: 27508348](Concise review of the safety and efficacy of drugs for bipolar disorder; states that "lithium remains the drug of choice for maintenance treatment of bipolar disoder"; no mention of hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review [Antipsychotics in bipolar disorders].[Encephale. 2004]Review [Antipsychotics in bipolar disorders].Vacheron-Trystram MN, Braitman A, Cheref S, Auffray L. Encephale. 2004 Sep-Oct; 30(5):417-24.
- Review Risperidone.[LiverTox: Clinical and Researc...]Review Risperidone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Lithium for acute mania.[Cochrane Database Syst Rev. 2019]Lithium for acute mania.McKnight RF, de La Motte de Broöns de Vauvert SJGN, Chesney E, Amit BH, Geddes J, Cipriani A. Cochrane Database Syst Rev. 2019 Jun 1; 6(6):CD004048. Epub 2019 Jun 1.
- Review Cidofovir.[LiverTox: Clinical and Researc...]Review Cidofovir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Association between mood-stabilizing medication and mental health resource use in the management of acute mania.[Psychiatr Serv. 1997]Association between mood-stabilizing medication and mental health resource use in the management of acute mania.Sajatovic M, Gerhart C, Semple W. Psychiatr Serv. 1997 Aug; 48(8):1037-41.
- Lithium - LiverToxLithium - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...