NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lefamulin is a new and relatively unique antibiotic used for treatment of community acquired pneumonia. Lefamulin has not been linked to an increased rate of transient serum liver test abnormalities during treatment or to instances of clinically apparent liver injury.
Background
Lefamulin (le fam’ ue lin) is a relatively new semisynthetic pleuromutilin derived antibiotic that has been shown to be effective in treating uncomplicated community acquired bacterial pneumonia. Lefamulin is a systemic pleuromutilin antibiotic and is believed to act via inhibition of bacterial ribosomal RNA, blocking critical protein synthesis. Lefamulin has bactericidal activity in vitro against S. pneumoniae, H. influenzae and M. pneumoniae including macrolide resistant strains and bacteriostatic activity against S. aureus [both methicillin-sensitive and -resistant] and S. pyogenes. Lefamulin is not active against Pseudomonas aeruginosa and Enterobacter species and has not been assessed clinically for activity against methicillin-resistant Staphylococcus aureus (MRSA) infections. In pivotal trials, lefamulin given for 5 to 7 days was effective as moxifloxacin in adults with community acquired pneumonia. Lefamulin was approved for this indication in 2019. Lefamulin is available in tablets of 600 mg for oral use and in single dose vials of 150 mg in 15 mL [for intravenous use [after dilution] under the brand name Xenleta. The recommended dose of lefamulin is 600 mg orally every 12 hours for 5 days or 150 mg intravenously (over 60 minutes) every 12 hours for 5 to 7 days. Side effects are usually mild-to-moderate in severity and can include diarrhea, nausea, vomiting and headache with oral doses, and injection site reactions and hyponatremia with intravenous use. Rare, but potentially serious adverse reactions include prolongation of the QTc interval, Clostridium difficile diarrhea and embryo-fetal toxicity.
Hepatotoxicity
Serum ALT, AST and GGT elevations above 3 times the upper limit of normal occurred in 1.2% to 3.5% of patients treated with lefamulin (n=641), but rates and severity of serum enzyme elevations were similar to those in comparator arms (moxifloxacin: n=641). These enzymes elevations were usually mild-to-moderate in severity, self-limited in duration and not accompanied by symptoms or jaundice. Instances of clinically apparent liver injury attributable to lefamulin have not been reported but this antibiotic has had limited general use.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which lefamulin might cause serum aminotransferase elevations or clinically apparent liver injury is not known, but it is metabolized by the liver via the hepatic microsomal P450 system (predominantly CYP 3A4) and liver injury might be due to production of a toxic or immunogenic intermediate of its metabolism. Concurrent use of inducers or inhibitors of CYP 3A4 can affect serum levels of lefamulin. Furthermore, serum levels of other CYP 3A4 substrates can be affected by concurrent use of lefamulin, which is of particular concern for substrates that, like lefamulin, prolong the QTc interval such as moxifloxacin, erythromycin, pimozide, antipsychotics and tricyclic antidepressants.
Outcome and Management
Serum enzyme elevations arise in a small percentage of patients being treated with lefamulin, but the course of therapy of this antibiotic is so limited that decisions to modify the dose or discontinue treatment early are unlikely to be practical or needed. Appearance of jaundice or symptoms of liver disease during therapy should lead to prompt discontinuation. There is no evidence to suggest cross sensitivity to liver injury or adverse events between lefamulin and other antibiotics.
Drug Class: Antiinfective Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lefamulin – Xenleta®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
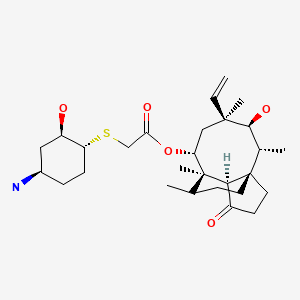
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lefamulin | 1061337-51-6 | C28-H45-N-O5-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2019
- Zimmerman HJ. Antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 611-21.(Extensive review of hepatotoxicity of antibiotics published in 1999 before the availability of lefamulin).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 468-9.(Review of hepatotoxicity of antibiotics published before the availability of lefamulin).
- MacDougall C. The quinolones. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1011-22.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/211672Orig1s000, %20211673Orig1s000MultidisciplineR.pdf . (FDA website with product labels and reviews of safety and efficacy of drugs that were the basis for their approval: safety analysis of laboratory findings are given on pages 189-90 of the multidisciplinary review). - Paukner S, Sader HS, Ivezic-Schoenfeld Z, Jones RN. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother. 2013;57:4489–95. [PMC free article: PMC3754340] [PubMed: 23836172](Analysis of the antibacterial spectrum of lefamulin showing broad activity against most gram positive- and selected gram-negative organisms).
- Eyal Z, Matzov D, Krupkin M, Paukner S, Riedl R, Rozenberg H, Zimmerman E, et al. A novel pleuromutilin antibacterial compound, its binding mode and selectivity mechanism. Sci Rep. 2016;6:39004. [PMC free article: PMC5154188] [PubMed: 27958389](Analysis of the crystal structure of lefamulin suggests that it binds to the peptidyl transferase center of bacterial ribosomal RNA, but not to human rRNA, leading to inhibition of bacterial but not mammalian protein synthesis).
- Veve MP, Wagner JL. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018;38:935–46. [PubMed: 30019769](Review of the structure, mechanism of action, spectrum of activity, clinical efficacy and safety of lefamulin, a novel antibiotic with activity against most gram positive bacteria; in discussion of safety mentions that “no clinically significant findings in any laboratory assessments… were reported”).
- Wicha WW, Prince WT, Lell C, Heilmayer W, Gelone SP. Pharmacokinetics and tolerability of lefamulin following intravenous and oral dosing. J Antimicrob Chemother. 2019;74 Supplement 3:iii19–iii26. [PMC free article: PMC6449572] [PubMed: 30949704](Human pharmacokinetic studies of lefamulin in 116 adults indicated that oral and intravenous dosing yielded comparable plasma levels, and side effects were mostly mild-to-moderate gastrointestinal symptoms; no mention of ALT levels or hepatotoxicity).
- Alexander E, Goldberg L, Das AF, Moran GJ, Sandrock C, Gasink LB, Spera P, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial. JAMA. 2019 Sep 27; [Epub ahead of print] [PMC free article: PMC6865224] [PubMed: 31560372](Among 738 adults with community acquired pneumonia treated with oral lefamulin [600 mg twice daily] or moxifloxacin [400 mg once daily] for 5 days, early clinical response rates were identical [91%], while gastrointestinal adverse events were more frequent with lefamulin including diarrhea [12% vs 1%] and nausea [5% vs 2%], but not ALT or AST elevations [0.5-0.8% vs 1.1%]).
- File TM Jr, Goldberg L, Das A, Sweeney C, Saviski J, Gelone SP, Seltzer E, et al. Efficacy and safety of iv-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: the phase 3 LEAP 1 trial. Clin Infect Dis. 2019 Feb 4; [Epub ahead of print] [PMC free article: PMC6853694] [PubMed: 30722059](Among 551 adults with community acquired pneumonia treated with intravenous lefamulin [150 mg every 12 hours] or moxifloxacin [400 mg every 24 hours] with option to switch to oral forms for 5-11 days, response rates were similar [87% vs 90%] as were adverse events overall [both 38%] and ALT elevations [1.8% vs 2.2%] and there were no hepatic related discontinuations or severe adverse events).
- Malani PN. Lefamulin-a new antibiotic for community-acquired pneumonia. JAMA. 2019 Sep 27; [Epub ahead of print] [PubMed: 31560367](Editorial in response to Alexander [2019] and File [2019], two trials that led to FDA approval of lefamulin, mentions that its spectrum of activity is similar to the fluoroquinolones, but it has more gastrointestinal side effects and is more expensive).
- Lefamulin (Xenleta) for community-acquired bacterial pneumonia. Med Lett Drugs Ther. 2019;61(1581):145–8. [PubMed: 31599865](Concise summary of the mechanism of action, clinical efficacy, safety and costs of lefamulin shortly after its approval in the US; does not mention serum enzyme elevations or hepatotoxicity but concludes that less expensive, older antibiotics with a longer history of safety and efficacy are preferred for treatment of community acquired pneumonia).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials.[Antibiotics (Basel). 2021]Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials.Paukner S, Mariano D, Das AF, Moran GJ, Sandrock C, Waites KB, File TM Jr. Antibiotics (Basel). 2021 Dec 4; 10(12). Epub 2021 Dec 4.
- Efficacy and Safety of Intravenous-to-oral Lefamulin, a Pleuromutilin Antibiotic, for the Treatment of Community-acquired Bacterial Pneumonia: The Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial.[Clin Infect Dis. 2019]Efficacy and Safety of Intravenous-to-oral Lefamulin, a Pleuromutilin Antibiotic, for the Treatment of Community-acquired Bacterial Pneumonia: The Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial.File TM, Goldberg L, Das A, Sweeney C, Saviski J, Gelone SP, Seltzer E, Paukner S, Wicha WW, Talbot GH, et al. Clin Infect Dis. 2019 Nov 13; 69(11):1856-1867.
- Review Lefamulin: A Novel Semisynthetic Pleuromutilin Antibiotic for Community-acquired Bacterial Pneumonia.[Clin Infect Dis. 2020]Review Lefamulin: A Novel Semisynthetic Pleuromutilin Antibiotic for Community-acquired Bacterial Pneumonia.Watkins RR, File TM. Clin Infect Dis. 2020 Dec 17; 71(10):2757-2762.
- Lefamulin efficacy and safety in a pooled phase 3 clinical trial population with community-acquired bacterial pneumonia and common clinical comorbidities.[BMC Pulm Med. 2021]Lefamulin efficacy and safety in a pooled phase 3 clinical trial population with community-acquired bacterial pneumonia and common clinical comorbidities.File TM Jr, Alexander E, Goldberg L, Das AF, Sandrock C, Paukner S, Moran GJ. BMC Pulm Med. 2021 May 8; 21(1):154. Epub 2021 May 8.
- Review A Review of Newly Approved Antibiotic Treatment for Community-Acquired Bacterial Pneumonia: Lefamulin.[Sr Care Pharm. 2020]Review A Review of Newly Approved Antibiotic Treatment for Community-Acquired Bacterial Pneumonia: Lefamulin.Wang H, Charles CV. Sr Care Pharm. 2020 Aug 1; 35(8):349-354.
- Lefamulin - LiverToxLefamulin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...