NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lasmiditan is a small molecule selective agonist of the serotonin 1F [5HT1F] receptor which decreases neuron activity that is thought to mediate pain and inflammation associated with migraine headaches. In clinical trials, lasmiditan was found to shorten the duration of migraine headache pain and other associated symptoms. Lasmiditan is generally well tolerated and has been associated with only rare instances of transient serum aminotransferase elevations during therapy but with no instances of clinically apparent liver injury.
Background
Lasmiditan (las mid’ i tan) is a selective, small molecule agonist of the serotonin 1F [5-HT1F] receptor that leads to a decrease in neuronal electrical activity in trigeminal neurons which is associated with migraine headaches. Lasmiditan is highly selective for the 5-HT1F receptor which is expressed on neurons in trigeminal ganglia and was developed as treatment of acute attacks of migraine with or without aura. In several randomized, placebo controlled trials, single doses of lasmiditan [50, 100 and 200 mg] increased the rate of being headache pain free 2 hours after dosing in comparison to placebo, as well as the rate of being free of the most bothersome other symptoms. Chronic therapy with lasmiditan had minimal effect in preventing migraine but it maintained its efficacy in treating acute episodes. Lasmiditan was approved for treatment of acute migraine with or without aura in adults in the United States in 2020, the first 5-HT1F agonist approved for this indication. Lasmiditan is available in tablets of 50 and 100 mg with the brand name Reyvow. The recommended dose is 50, 100 or 200 mg orally as soon as possible after onset of migraine. The dose should not be repeated during any 24 hour period. Lasmiditan can be used by patients receiving preventive therapies for migraine, but is not approved for chronic use as a means of preventing migraine headaches. Lasmiditan has central nervous system side effects such as dizziness, impaired balance, somnolence, fatigue, sedation and impairment of driving (which should be avoided for at least 8 hours after dosing). Palpitations, bradycardia and increase in blood pressure can also occur, but severe adverse reactions are rare. Unlike triptans, which act on 5-HTB/D receptors, lasmitidan does not induce vasoconstriction and can be used in patients with coronary artery disease. Rare but potentially serious adverse events include bradycardia (particularly with other drugs that slow the heart rate), the serotonin syndrome and hypersensitivity reactions with rash and angioedema.
Hepatotoxicity
In preregistration controlled trials of lasmiditan in several thousand patients, mild-to-moderate serum aminotransferase elevations arose in a small percentage of patients (1% or less) and overall rates were not different from those in placebo recipients. In the controlled trials and subsequently with general use, there have been no reports of liver injury with symptoms or jaundice attributed to lasmiditan.
Likelihood score: E (unlikely cause of clinically apparent acute liver injury).
Mechanism of Injury
Possible mechanisms of liver injury due to lasmiditan are not known. It is metabolized by multiple enzyme systems but does not have major CYP 450 interactions. It is generally given intermittently in single doses which may account for its relative freedom from major serious adverse events.
Outcome and Management
Drug Class: Migraine Headache Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lasmiditan – Reyvow®
DRUG CLASS
Migraine Headache Agents
Product labeling at DailyMed, National Library of Medicine, NIH
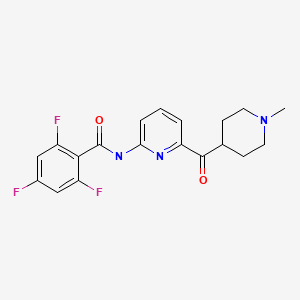
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lasmiditan | 439239-90-4 | C19-H18-F3-N3-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 July 2021
Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin).
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of lasmiditan).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/211280Orig1s000MedR.pdf. (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA multidisciplinary scientific review of the lasmiditan application including specific discussion of hepatic adverse events, found similar low rates of significant serum ALT and AST elevations [<1%] with lasmiditan therapy as with placebo and concluded that there was no signal of hepatotoxicity identified). - Ferrari MD, Färkkilä M, Reuter U, Pilgrim A, Davis C, Krauss M, Diener HC., European COL-144 Investigators. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan--a randomised proof-of-concept trial. Cephalalgia. 2010;30:1170–8. [PubMed: 20855362](Among 130 patients with an acute migraine enrolled in a placebo controlled study of escalating doses of intravenous lasmiditan, improvements in headache pain occurred within 2 hours with single doses of 10, 20 and 45 mg and adverse events included dizziness, paresthesias and fatigue, but there were no serious adverse events and “no clinically significant changes in … clinical chemistry parameters”).
- Färkkilä M, Diener HC, Géraud G, Láinez M, Schoenen J, Harner N, Pilgrim A, et al. COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405–13. [PubMed: 22459549](Among 391 patients treated in a randomized controlled dose-finding trial for acute migraine with lasmiditan [5, 100, 200 or 400 mg] vs placebo, freedom from headache pain at 2 hours was achieved by more patients on higher doses of lasmiditan than placebo [14% to 28% vs 7%], but adverse events were more frequent with higher doses as well [65-86% vs 22%] including dizziness, fatigue, vertigo, paresthesias, somnolence and nausea).
- Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB., COL MIG-301 Study Group. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology. 2018;91:e2222–e2232. [PMC free article: PMC6329326] [PubMed: 30446595](Among 1856 adults with an acute migraine attack in a randomized controlled trial, those treated with a single dose of lasmiditan [100 or 200 mg] were more likely to be headache pain free at 2 hours than those on placebo [28% and 32% vs 15%] and were more likely to be free of their most bothersome other symptom [41% and 41% vs 29.5%], and although overall adverse event rates were more frequent with drug [36% and 43% vs 16%], there were no drug related serious adverse events and “no clinically meaningful differences in … blood chemistry …across treatment groups”).
- Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK, Gaul C. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142:1894–904. [PMC free article: PMC6620826] [PubMed: 31132795](Among 3005 patients treated in a randomized controlled trial, patients with an acute attack of migraine were more likely to be headache pain free two hours after receiving lasmiditan [50, 100 or 200 mg] than placebo [29%, 31% and 39% vs 21%] and more likely to be free of the other most bothersome symptom [41%, 44% and 49% vs 36%] but were also more likely to have an adverse event [25%; 36% and 39% vs 12%], although there “were no clinically meaningful differences in… blood chemistry… across the treatment groups).
- Krege JH, Rizzoli PB, Liffick E, Doty EG, Dowsett SA, Wang J, Buchanan AS. Safety findings from Phase 3 lasmiditan studies for acute treatment of migraine: Results from SAMURAI and SPARTAN. Cephalalgia. 2019;39:957–66. [PMC free article: PMC6787764] [PubMed: 31166697](In a secondary analysis of adverse event rates from two large, randomized trials of oral lasmiditan [50, 100 or 200 mg; n=3177] versus placebo [n=1262] for acute migraine [Kuca 2018, Goadsby 2019], there were no deaths and serious adverse event rates were similar in all groups [0.2%], while there was a dose related increase in overall adverse event rates [13.5% with placebo vs 25%, 36% and 41% with lasmiditan] and “no significant differences in frequency of abnormal hepatic laboratory results” including serum ALT, AST, alkaline phosphatase and bilirubin levels).
- Loo LS, Ailani J, Schim J, Baygani S, Hundemer HP, Port M, Krege JH. Efficacy and safety of lasmiditan in patients using concomitant migraine preventive medications: findings from SAMURAI and SPARTAN, two randomized phase 3 trials. J Headache Pain. 2019;20:84. [PMC free article: PMC6734212] [PubMed: 31340760](Post-hoc analysis of two large controlled trials of lasmiditan vs placebo for acute migraine [Kuca 2018, Goadsby 2019] for patients concurrently taking migraine preventive treatments [such as valproate, topiramate, beta-blockers, antidepressants, botulinum toxin type A and candesartan] had similar rates of clinical response as well as side effects as those not taking preventive treatments).
- Brandes JL, Klise S, Krege JH, Case M, Khanna R, Vasudeva R, Raskin J, et al. Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study). Cephalalgia. 2019;39:1343–57. [PMC free article: PMC6779019] [PubMed: 31433669](Among 1978 patients with migraine headaches who participated in controlled trials of single doses of lasmiditan who were then enrolled in a long term open-label study of 100 vs 200 mg of lasmiditan for each new attack, clinical response rates and adverse event rates were similar to previous reports and overall “no pattern of clinically meaningful changes from baseline were observed in laboratory parameters”).
- Lamb YN. Lasmiditan: first approval. Drugs. 2019;79:1989–96. [PubMed: 31749059](Review of the mechanism of action, history of development, pharmacology, clinical efficacy and safety of lasmiditan shortly after its approval in the US, discusses common adverse events of dizziness, paresthesia, somnolence, nausea, fatigue, weakness and hypesthesia, and the rare instances of serotonin syndrome and hypersensitivity reactions, but does not mention ALT elevations or hepatotoxicity).
- Lasmiditan (Reyvow) and ubrogepant (Ubrelvy) for acute treatment of migraine. Med Lett Drugs Ther. 2020;62(1593):35–9. [PubMed: 32555120](Concise review of the mechanism of action, clinical efficacy, safety and costs of ubrogepant and lasmiditan as therapy of acute migraine shortly after their approval for this indication in the US, mentions side effects of lasmiditan being dizziness, paresthesias, fatigue, nausea and somnolence but does not mention ALT elevations or hepatotoxicity).
- Drugs for migraine. Med Lett Drugs Ther. 2020;62(1608):153–60. [PubMed: 33434187](Concise summary of the relative clinical efficacy, safety and costs of drugs to treat acute migraine headache [such as analgesics, opiates, triptans, ergots and oral CGRP receptor antagonists] and to prevent migraines [such as anticonvulsants, beta blockers, antidepressants and the monoclonal antibodies to CGRP and its receptor]).
- Ashina M, Reuter U, Smith T, Krikke-Workel J, Klise SR, Bragg S, Doty EG, et al. Randomized, controlled trial of lasmiditan over four migraine attacks: Findings from the CENTURION study. Cephalalgia. 2021;41:294–304. [PMC free article: PMC7961651] [PubMed: 33541117](Among 1471 patients with migraine headaches randomized to lasmiditan [50, 100 or 200 mg] vs placebo for up a 4 acute attacks, the response rate by dose was consistent over the four attacks while adverse event rates decreased; and “Overall, the changes in laboratory values were similar across treatment groups”).
- Robbins MS. Diagnosis and management of headache: a review. JAMA. 2021;325:1874–85. [PubMed: 33974014](Review of the diagnosis and management of headache including use of lasmiditan for acute migraine attacks which has side effects of dizziness, paresthesias, fatigue, nausea and somnolence, and patients should not drive or use heavy equipment for 8 hours after taking it; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Novel Therapies in Acute Migraine Management: Small-Molecule Calcitonin Gene-Receptor Antagonists and Serotonin 1F Receptor Agonist.[Ann Pharmacother. 2021]Novel Therapies in Acute Migraine Management: Small-Molecule Calcitonin Gene-Receptor Antagonists and Serotonin 1F Receptor Agonist.Joyner KR, Morgan KW. Ann Pharmacother. 2021 Jun; 55(6):745-759. Epub 2020 Sep 29.
- Onset of Efficacy Following Oral Treatment With Lasmiditan for the Acute Treatment of Migraine: Integrated Results From 2 Randomized Double-Blind Placebo-Controlled Phase 3 Clinical Studies.[Headache. 2019]Onset of Efficacy Following Oral Treatment With Lasmiditan for the Acute Treatment of Migraine: Integrated Results From 2 Randomized Double-Blind Placebo-Controlled Phase 3 Clinical Studies.Ashina M, Vasudeva R, Jin L, Lombard L, Gray E, Doty EG, Yunes-Medina L, Kinchen KS, Tassorelli C. Headache. 2019 Nov; 59(10):1788-1801. Epub 2019 Sep 17.
- Review The 5-HT1F receptor agonist lasmiditan as a potential treatment of migraine attacks: a review of two placebo-controlled phase II trials.[J Headache Pain. 2012]Review The 5-HT1F receptor agonist lasmiditan as a potential treatment of migraine attacks: a review of two placebo-controlled phase II trials.Tfelt-Hansen PC, Olesen J. J Headache Pain. 2012 Jun; 13(4):271-5. Epub 2012 Mar 20.
- Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials.[J Headache Pain. 2019]Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials.Shapiro RE, Hochstetler HM, Dennehy EB, Khanna R, Doty EG, Berg PH, Starling AJ. J Headache Pain. 2019 Aug 29; 20(1):90. Epub 2019 Aug 29.
- Review The safety and efficacy of the 5-HT 1F receptor agonist lasmiditan in the acute treatment of migraine.[Expert Opin Pharmacother. 2017]Review The safety and efficacy of the 5-HT 1F receptor agonist lasmiditan in the acute treatment of migraine.Raffaelli B, Israel H, Neeb L, Reuter U. Expert Opin Pharmacother. 2017 Sep; 18(13):1409-1415. Epub 2017 Aug 10.
- Lasmiditan - LiverToxLasmiditan - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...