NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ixabepilone is a semisynthetic epothilone analogue that acts to stabilize microtubules thereby preventing mitosis and causing growth arrest in cancer cells. Ixabepilone is approved for use in refractory cases of advanced breast cancer. Its use is associated with a low rate of serum enzyme elevation, but ixabepilone has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Ixabepilone (ix" a bep' i lone) is semisynthetic analogue of epothilone B, epothilones being cytotoxic macrolides that cause cell growth arrest by binding to microtubules and preventing mitosis. Ixabepilone is similar in activity to taxanes, but differs structurally and is not affected by taxane resistance. Therapy with ixabepilone has been shown to prolong relapse-free as well as overall survival in women with locally advanced or metastatic breast cancer that has not responded or has relapsed after standard therapies such as with taxanes, capecitabine and anthracyclines. Ixabepilone was approved for use in the United States in 2007 and current indications are for locally advanced or metastatic breast cancer given as monotherapy or in combination with capecitabine after failure of other treatments. Ixabepilone is available in vials of 15 and 45 mg supplied with diluent under the brand name Ixempra. The recommended starting dose is 40 mg/m2 intravenously [infused over 3 hours] every 3 weeks. Common side effects include peripheral neuropathy, fatigue, muscle and joint pains, stomatitis, diarrhea, anorexia, weight loss, constipation, hair loss, hand-foot syndrome and myelosuppression. Rare, but potentially serious side effects include severe peripheral neuropathy, neutropenia, hypersensitivity reactions and fetal toxicity. Toxicity of ixabepilone is greater in patients with preexisting liver abnormalities which should trigger use of lower doses when given as monotherapy and avoidance of its use in combination with capecitabine.
Hepatotoxicity
In preregistration controlled trials, serum aminotransferase elevations and other liver test abnormalities were rarely mentioned. A high proportion of patients treated had mild-to-moderate serum enzyme elevations at the time of starting ixabepilone, probably because of hepatic metastases and the use of other antineoplastic agents. During ixabepilone therapy, worsening of serum enzyme elevations occurred in up to 15% of patients, but ALT elevations above 5 times the upper limit of normal were rare, and there were no reports of severe hepatic adverse events or discontinuations because of enzyme elevations or clinically apparent liver disease. Nevertheless, jaundice and acute liver failure as well as elevations in serum ALT, AST, alkaline phosphatase, and bilirubin are mentioned as occurring in clinical trials in the product label. Since the approval and more widescale use of ixabepilone, there have been no publications or descriptions of the clinical features of hepatotoxicity with jaundice associated with its use. Thus, clinically apparent liver injury probably occurs in a small proportion of patients receiving ixabepilone, but its relationship with the drug is unclear.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver enzyme elevations that occur during enzalutamide therapy is unknown. Ixabepilone is extensively metabolized in the liver predominantly by CYP 3A4 and 2D6 and is a strong inducer of CYP 3A4 and a moderate inducer of 2D6. Ixabepilone is susceptible to drug-drug interactions with inhibitors, inducers or substrates of these microsomal enzymes.
Outcome and Management
The liver injury linked to ixabepilone therapy has been generally mild, consisting of transient and asymptomatic elevations in serum aminotransferase levels. Ixabepilone has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between ixabepilone and other therapies of advanced or metastatic breast cancer.
Drug Class: Antineoplastic Agents, Breast Cancer Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ixabepilone – Ixempra®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ixabepilone | 219989-84-1 | C27-H42-N2-O5-S |
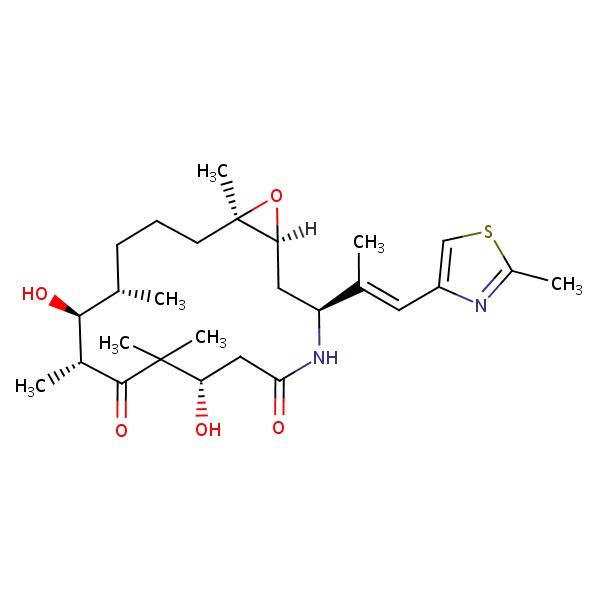 |
ANNOTATED BIBLIOGRAPHY
References updated: 05 January 2017
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 before the availability of ixabepilone).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; ixabepilone is not discussed).
- Moy B, Lee RJ, Smith M. Hormone therapy in prostate cancer. Natural products in cancer chemotherapy: hormones and related agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1763-9.(Textbook of pharmacology and therapeutics).
- Low JA, Wedam SB, Lee JJ, Berman AW, Brufsky A, Yang SX, Poruchynsky MS, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol 2005; 23: 726-34. [PubMed: 15837987](Among 37 women with metastatic breast cancer not responding to taxanes who were treated with ixabepilone [6 mg/m2 daily for 5 days every 3 weeks], the objective response rate was 22% and moderate-to-severe side effects included neutropenia, fatigue, diarrhea, nausea, myalgia and neuropathy; there were no liver related discontinuations).
- Thomas E, Tabernero J, Fornier M, Conté P, Fumoleau P, Lluch A, Vahdat LT, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol 2007; 25: 3399-406. [PubMed: 17606975](Among 49 women with refractory, metastatic breast cancer treated with ixabepilone, the overall response rate was 12% [partial responses only] and toxicities were frequent, but "manageable and mostly mild"; "hepatic" adverse events occurred in 6% of patients, but no details provided).
- Vansteenkiste J, Lara PN Jr, Le Chevalier T, Breton JL, Bonomi P, Sandler AB, Socinski MA, et al. Phase II clinical trial of the epothilone B analog, ixabepilone, in patients with non small-cell lung cancer whose tumors have failed first-line platinum-based chemotherapy. J Clin Oncol 2007; 25: 3448-55. [PubMed: 17606973](Among 201 patients with non-small cell lung cancer who were refractory to standard therapy and were treated with ixabepilone, objective response rates were low and adverse side effects were common; no mention of ALT elevations or hepatotoxicity).
- Denduluri N, Low JA, Lee JJ, Berman AW, Walshe JM, Vatas U, Chow CK, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol 2007; 25: 3421-7. [PubMed: 17606971](Among 23 women with refractory metastatic breast cancer treated with ixabepilone [6 mg/m2 for 5 days every 3 weeks], partial response rates were 57% and side effects included neuropathy, fatigue and neutropenia; no mention of ALT elevations or hepatotoxicity).
- Roché H, Yelle L, Cognetti F, Mauriac L, Bunnell C, Sparano J, Kerbrat P, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol 2007; 25: 3415-20. [PubMed: 17606972](Among 65 women with advanced, refractory breast cancer treated with ixabepilone [40 mg/m2 every 3 weeks], the objective response rate was 42% and most adverse events were "manageable"; no mention of ALT elevations or hepatotoxicity).
- Perez EA, Lerzo G, Pivot X, Thomas E, Vahdat L, Bosserman L, Viens P, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol 2007; 25: 3407-14. [PubMed: 17606974](Among 126 patients with advanced, refractory breast cancer treated with ixabepilone [40 mg/m2 every 3 weeks] for 1 to 16 cycles, the objective response rate was 12% and toxicity was "manageable and primarily" mild-to-moderate; no mention of ALT elevations or hepatotoxicity).
- Pivot X, Dufresne A, Villanueva C. Efficacy and safety of ixabepilone, a novel epothilone analogue. Clin Breast Cancer 2007; 7: 543-9. [PubMed: 17509162](Review of the development, structure, mechanism of action, preclinical and clinical efficacy and side effects, focusing upon neuropathy, myelosuppression, and hypersensitivity reactions; no mention of ALT elevations or hepatotoxicity).
- Bunnell C, Vahdat L, Schwartzberg L, Gralow J, Klimovsky J, Poulart V, Peck R, et al. Phase I/II study of ixabepilone plus capecitabine in anthracycline-pretreated/resistant and taxane-resistant metastatic breast cancer. Clin Breast Cancer 2008; 8: 234-41. [PubMed: 18650153](Among 106 women with metastatic, refractory breast cancer treated with ixabepilone and capecitabine in two different dose schedules, the overall response rate was 30% and 47% and side effects included hand foot syndrome, fatigue, myalgia, nausea, neuropathy, diarrhea and neutropenia; no mention of ALT elevations or hepatotoxicity).
- Ixabepilone (Ixempra) for breast cancer. Med Lett Drugs Ther 2008; 50 (1278): 7-8. [PubMed: 18219261](Concise review of the mechanism of action, clinical efficacy, safety and costs of ixabepilone as therapy of breast cancer shortly after its approval in the US; mentions side effects of peripheral neuropathy, fatigue, myalgia, alopecia, nausea, stomatitis, diarrhea and muscle pain as well as episodes of neutropenia).
- Nimeiri HS, Singh DA, Kasza K, Taber DA, Ansari RH, Vokes EE, Kindler HL. The epothilone B analogue ixabepilone in patients with advanced hepatobiliary cancers: a trial of the University of Chicago Phase II Consortium. Invest New Drugs 2010; 28: 854-8. [PMC free article: PMC4635688] [PubMed: 19669700](Among 54 patients with advanced hepatobiliary cancers treated with ixabepilone [40 mg/m2 every 3 weeks], the objective response rate was only 8.5% and severe side effects included neutropenia, fatigue, hypersensitivity reactions and sensory neuropathy; no mention of serum enzyme elevations or worsening of liver disease).
- Huang H, Menefee M, Edgerly M, Zhuang S, Kotz H, Poruchynsky M, Huff LM, et al. A phase II clinical trial of ixabepilone (Ixempra; BMS-247550; NSC 710428), an epothilone B analog, in patients with metastatic renal cell carcinoma. Clin Cancer Res 2010; 16: 1634-41. [PMC free article: PMC7006234] [PubMed: 20179242](Among 87 patients with metastatic renal cell carcinoma treated with ixabepilone, the overall response rate was 13% and adverse events included neuropathy [59%], alopecia [70%], severe neutropenia [11%] and febrile neutropenia [3.4%], no mention of ALT elevations or hepatotoxicity).
- McMeekin S, Dizon D, Barter J, Scambia G, Manzyuk L, Lisyanskaya A, Oaknin A, et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in 9 with advanced endometrial cancer. Gynecol Oncol 2015; 138: 18-23. [PubMed: 25925990](Among 496 women with advanced or metastatic endometrial cancer treated with ixabepilone or standard therapy [doxorubicin or paclitaxel] every 21 days, time of objective response was less with ixabepilone [10.3 vs 13.3 months] and adverse events were more frequent including peripheral neuropathy [44% vs 26%]; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Mechanism of action of ixabepilone and its interactions with the βIII-tubulin isotype.[Cancer Chemother Pharmacol. 2015]Mechanism of action of ixabepilone and its interactions with the βIII-tubulin isotype.Lopus M, Smiyun G, Miller H, Oroudjev E, Wilson L, Jordan MA. Cancer Chemother Pharmacol. 2015 Nov; 76(5):1013-24. Epub 2015 Sep 28.
- Review Efficacy and safety of ixabepilone, a novel epothilone analogue.[Clin Breast Cancer. 2007]Review Efficacy and safety of ixabepilone, a novel epothilone analogue.Pivot X, Dufresne A, Villanueva C. Clin Breast Cancer. 2007 Apr; 7(7):543-9.
- In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models.[Clin Cancer Res. 2005]In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models.Peterson JK, Tucker C, Favours E, Cheshire PJ, Creech J, Billups CA, Smykla R, Lee FY, Houghton PJ. Clin Cancer Res. 2005 Oct 1; 11(19 Pt 1):6950-8.
- Chemotherapy reaction induced by ixabepilone, a microtubule stabilizing agent, mimicking extramammary Paget's disease in a patient with breast carcinoma.[J Cutan Pathol. 2016]Chemotherapy reaction induced by ixabepilone, a microtubule stabilizing agent, mimicking extramammary Paget's disease in a patient with breast carcinoma.Millsop JW, Sharon VR, Petukhova T, Fung MA, Kiuru M. J Cutan Pathol. 2016 Dec; 43(12):1215-1219. Epub 2016 Oct 24.
- Review Ixabepilone: a novel microtubule-stabilizing agent for the treatment of metastatic breast cancer.[Am J Health Syst Pharm. 2008]Review Ixabepilone: a novel microtubule-stabilizing agent for the treatment of metastatic breast cancer.Goodin S. Am J Health Syst Pharm. 2008 Nov 1; 65(21):2017-26.
- Ixabepilone - LiverToxIxabepilone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...