NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Infigratinib is a FGF receptor kinase inhibitor that is used in the treatment of unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma. Infigratinib is associated with transient and usually mild elevations in serum aminotransferase during therapy, but has not been convincingly linked to cases of clinically apparent liver injury.
Background
Infigratinib (fue” ti ba’ ti nib) is an orally available, specific inhibitor of the fibroblast growth factor (FGF) receptor 1, 2 and 3 kinases, that is used in the therapy advanced or metastatic cholangiocarcinoma. Aberrant FGF receptor signaling is associated with several cancer types in which amplifications, translocations, fusings, and other activating point mutations of FGF receptors are found. Cholangiocarcinomas often harbor mutants of the FGF receptor 2 that cause the activation of FGF pathways that promote uncontrolled growth and tumorigenesis. Inhibition of the mutant receptor causes regression of cell proliferation and promotes cell death. Studies in cell culture and in animal models suggested that infigratinib was active in inhibiting cancer cell growth. Trials in patients with cholangiocarcinoma demonstrated a significant overall durable response rate, for which reason infigratinib received accelerated approval for use in the United States in 2021. Current indications are limited to adults with previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma with FGF receptor 2 gene fusions or other rearrangements. Infigratinib continues to be under evaluation for its long term safety and efficacy in cholangiocarcinoma and for its possible efficacy in other forms of cancer associated with FGF receptor gene alternations. Infigratinib is available in capsules of 25 and 100 mg under the brand name Truseltiq. The recommended dose is 125 mg once daily for 21 days of a 28 day cycle, continued indefinitely or until there is disease progression or unacceptable toxicity. Adverse events are common and include hyperphosphatemia (an effect of inhibition of FGF signaling), as well as symptoms of fatigue, nausea, diarrhea, constipation, dysgeusia, anorexia, abdominal pain, musculoskeletal pain, dizziness, alopecia, dryness of the eyes, mouth and skin, blurred vision, nail toxicity, and hand-foot syndrome. Severe adverse events include severe hyperphosphatemia, ocular toxicity, infections and sepsis, and embryo-fetal toxicity.
Hepatotoxicity
In the open label clinical trials of infigratinib for advanced or metastatic cholangiocarcinoma, adverse events were common and led to dose interruptions in 64%, dose reductions in 60% of patients, and permanent discontinuations in 15% largely for hyperphosphatemia, infections and sepsis rather than liver injury. In preregistration trials in 108 patients, ALT elevations arose in 51% and to above 5 times ULN in 6%. The elevations were typically self-limited and resolved rapidly with or without dose adjustments. No patients developed clinically apparent liver injury or jaundice. Since its approval, there have been no reports clinically apparent liver injury attributed to infigratinib. However, the total clinical experience with its use has been limited and the frequency of serum aminotransferase elevations during therapy suggest that clinically significant liver injury may occur.
Likelihood score: E* (unproven but possible, rare cause of clinically apparent liver injury).
Mechanism of Injury
The causes of serum enzyme elevations or liver injury from infigratinib therapy are not known. Some of the adverse effects of infigratinib are due to its effects on FGF signaling (hyperphosphatemia) and others may be due to off-target effects on other kinases. Infigratinib is metabolized in the liver largely by CYP 3A4 and liver injury might be caused by production of a toxic or immunogenic intermediate. Because it is a substrate for CYP 3A4, infigratinib is susceptible to drug-drug interactions with agents that inhibit or induce this specific hepatic microsomal activity.
Outcome and Management
Infigratinib is associated with a moderate rate of serum aminotransferase elevations that are generally transient and not associated with symptoms or jaundice. While regular monitoring of liver tests is not specifically recommended during infigratinib therapy, elevations if confirmed should lead to more careful follow up monitoring. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) or any elevations accompanied by jaundice or symptoms should lead to dose reduction or temporary cessation until the abnormalities resolve or an alternative cause is identified. There is no evidence to suggest a cross reactivity in risk for adverse events, hypersensitivity or hepatic injury between infigratinib and other protein kinases or FGF receptor inhibitors.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Other FGF Receptor Kinase Inhibitors: Erdafitinib, Futibatinib, Pemigatinib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Infigratinib – Truseltiq®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
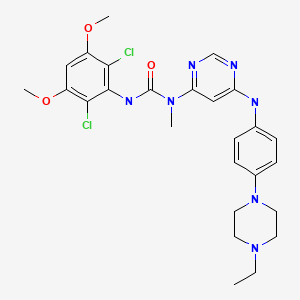
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Infigratinib | 872511-34-7 | C26-H31-Cl2-N7-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 November 2022
Abbreviations: FGF, fibroblast growth factor.
- Zimmerman HJ. Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase inhibitors such as infigratinib).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents discusses several tyrosine kinase inhibitors including imatinib, gefitinib, erlotinib and crizotinib, but not the FGF receptor inhibitors).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway-targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2021/214622Orig1s000MultidisciplineR.pdf. (FDA website with product labels, letters and multidisciplinary review of the applications for infigratinib mentions that adverse events were common, led to drug discontinuation in 15% [none liver related], interruptions in 64% and dose reductions in 60%; ALT elevations arose in 51% of 108 treated subjects which were above 5 times ULN in 6%, but all were self-limited and there were no deaths from liver failure, serious hepatic adverse events or instances of clinically apparent liver injury with jaundice). - Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491–503. [PubMed: 23620168](Review of the hepatotoxicity of 18 tyrosine kinase inhibitors approved for use in cancer in the US as of 2013, before the availability of infigratinib which is not discussed).
- Guagnano V, Furet P, Spanka C, Bordas V, Le Douget M, Stamm C, Brueggen J, et al. Discovery of3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem. 2011;54:7066–83. [PubMed: 21936542](Description of the discovery of a series of FGF receptor inhibitors and selection of infigratinib for its in vitro and in vivo effects in inhibition of FGF receptor 1, 2 and 3 activity and inhibition of growth and proliferation of cancer cell lines that overexpressed FGF receptors).
- Arai Y, Totoki Y, Hosoda F, Shirota T, Hama N, Nakamura H, Ojima H, et al. Fibroblast growth factor receptor tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–34. [PubMed: 24122810](Whole transcriptome sequencing from 8 cholangiocarcinomas identified two FGF receptor fusion genes and screening of different human cancer tissues found FGF receptor fusions in 9 of 102 cholangiocarcinomas [all intrahepatic] but only 1 of 149 colorectal, 1 of 96 hepatocellular, and none of 212 gastric cancers; expression of the fusion kinases in cell cultures led to anchorage independent growth and tumorigenesis when implanted in mice).
- Nogova L, Sequist LV, Perez Garcia JM, Andre F, Delord JP, Hidalgo M, Schellens JH, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol. 2017;35:157–165. [PMC free article: PMC6865065] [PubMed: 27870574](In phase 1 dose escalating studies of infigratinib in 132 patients with advanced, refractory cancers harboring FGF receptor mutations or fusions, the maximum tolerated dose was 125 mg daily and dose defining toxicities included aminotransferase elevations; a regimen of 21 days on and 7 days off was better tolerated that continuous therapy).
- Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36:276–282. [PMC free article: PMC6075847] [PubMed: 29182496](Among 61 patients with advanced or metastatic cholangiocarcinoma harboring FGF receptor alterations treated with infigratinib [125 mg daily for 21 days in 28 day cycles], the objective response rate was 15%, occurring only in patients with FGF receptor fusions, while adverse events included hyperphosphatemia in 81%, fatigue 36%, stomatitis 30%, alopecia 26%, hand-foot syndrome 21%, ALT elevations 10%, and hepatic failure in 1 patient [3%]).
- Hartley IR, Miller CB, Papadakis GZ, Bergwitz C, Del Rivero J, Blau JE, Florenzano P, et al. Targeted FGFR blockade for the treatment of tumor-induced osteomalacia. N Engl J Med. 2020;383:1387–1389. [PMC free article: PMC7561341] [PubMed: 32905668](66 year old man with mesenchymal tumor that produced FGF 23 resulting in hypophosphatemia and severe osteomalacia, was treated with infigratinib with a dramatic clinical and biochemical response followed by a relapse when infigratinib was stopped).
- Krook MA, Reeser JW, Ernst G, Barker H, Wilberding M, Li G, Chen HZ, et al. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 2021;124:880–892. [PMC free article: PMC7921129] [PubMed: 33268819](Review of the association of FGF receptors and cancer chemotherapeutic agents, activating mutations being found in 5% to 10% of cancers overall, but in 10% to 20% of cholangiocarcinomas).
- Kang C. Infigratinib: first approval. Drugs. 2021;81:1355–1360. [PMC free article: PMC8610935] [PubMed: 34279850](Review of the chemical structure, mechanism of action, pharmacokinetics, clinical efficacy and safety of infigratinib shortly after its approval in the US, mentions ALT and AST elevations in list of adverse events but not clinically apparent hepatotoxicity or jaundice).
- Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, Waldschmidt DT, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6:803–815. [PubMed: 34358484](Among 108 patients with advanced or metastatic cholangiocarcinoma with FGF2 fusions or rearrangements treated with infigratinib [125 mg for 21 of 28 day cycles], the objective response rate was 23% and adverse events included hyperphosphatemia [77%], stomatitis [55%], fatigue [40%], alopecia [38%], dry eyes [34%], and ALT elevations [15% with 2% above 5 times ULN], but there were no instances of clinically apparent liver injury with jaundice).
- Neuzillet C. Infigratinib in pretreated cholangiocarcinoma with FGFR2 fusions or rearrangements. Lancet Gastroenterol Hepatol. 2021;6:773–775. [PubMed: 34358483](Editorial in response to Javle et al [2021] comparing results of second-line infigratinib therapy of advanced or metastatic cholangiocarcinoma [objective response rate of 23.1%] with the previous standard chemotherapy [FOLFOX: 7.7%] and the similar FGF receptor kinase inhibitor pemigatinib [35.5%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Futibatinib.[LiverTox: Clinical and Researc...]Review Futibatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pemigatinib.[LiverTox: Clinical and Researc...]Review Pemigatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study.[Lancet Gastroenterol Hepatol. ...]Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study.Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, Waldschmidt DT, Goyal L, Borbath I, El-Khoueiry A, et al. Lancet Gastroenterol Hepatol. 2021 Oct; 6(10):803-815. Epub 2021 Aug 3.
- Review Infigratinib for cholangiocarcinoma.[Drugs Today (Barc). 2022]Review Infigratinib for cholangiocarcinoma.Sadeghi S. Drugs Today (Barc). 2022 Jul; 58(7):327-334.
- Review Targeted Therapy for Advanced or Metastatic Cholangiocarcinoma: Focus on the Clinical Potential of Infigratinib.[Onco Targets Ther. 2021]Review Targeted Therapy for Advanced or Metastatic Cholangiocarcinoma: Focus on the Clinical Potential of Infigratinib.Yu J, Mahipal A, Kim R. Onco Targets Ther. 2021; 14:5145-5160. Epub 2021 Oct 23.
- Infigratinib - LiverToxInfigratinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...