NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Guanfacine is a selective alpha-adrenergic receptor agonist used for the treatment of hypertension and attention deficit hyperactivity disorder (ADHD) in adults and children. Guanfacine has not been linked to serum enzyme elevations during treatment or to cases of acute, clinically apparent liver injury.
Background
Guanfacine (gwahn' fa seen) is a selective alpha-2A-adrenergic receptor agonist initially approved as therapy of hypertension and subsequently for management of attention deficit hyperactivity disorder (ADHD). It is currently used mostly as therapy of ADHD, both in children and adults. Therapy with extended release formulations of guanfacine have been shown to lead to improvements in levels of psychological functioning and performance in children and adolescents with suspected ADHD. Guanfacine is available in multiple formulations including tablets of 1 and 2 mg generically and under the brand name Tenex for treatment of hypertension and as extended release tablets of 1, 2, 3 and 4 mg generically and under the brand name Intuniv for the treatment of ADHD. The recommended initial dosage for hypertension in adults is 1 mg daily, with subsequent increases to a maintenance dose of up to 3 mg daily. The recommended dosage of the extended release formulation for treatment of ADHD is 1 mg daily, with subsequent increases to a maintenance dose of up to 7 mg daily. Common side effects include somnolence, sedation, headache, nausea, dizziness, bradycardia, low blood pressure, fatigue and dry mouth. Rare, but potentially severe adverse events include skin rash, bradycardia, hypotension and syncope.
Hepatotoxicity
In the multiple clinical trials of guanfacine in adolescents and children with ADHD there were no reports of serum enzyme elevations or in instances of clinically apparent liver injury. Furthermore, despite widescale use of the agent for both hypertension and ADHD, there have been no reports of clinically apparent liver injury attributable to guanfacine. Thus, significant liver injury from guanfacine must be quite rare, if it exists at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which guanfacine might cause liver injury is unknown. Guanfacine is metabolized in the liver by the cytochrome P450 system, predominantly CYP 3A4 and production of a toxic intermediate or immunogenic byproduct are reasonable explanations. Guanfacine is susceptible to drug-drug interactions with agents that modulate CYP 3A4 activity, inhibitors causing an increase and inducers causing a decrease in guanfacine plasma levels. The hepatic safety of guanfacine may relate to the low total daily doses used (1 to 7 mg daily).
Drug Class: Antihypertensive Agents, ADHD Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Guanfacine – Generic, Tenex®, Intuniv®
DRUG CLASS
Antihypertensive Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
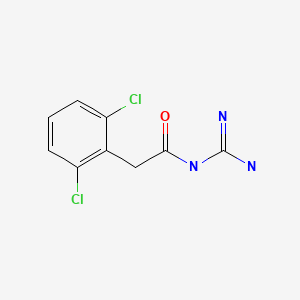
| Guanfacine | 29110-47-2 | C9-H9-Cl2-N3-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 August 2021
Abbreviations: ADHD, attention deficit/hyperactivity disorder.
- Zimmerman HJ. Psychotropic and anticonvulsant agents. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-516.(Expert review of hepatotoxicity published in 1999; guanfacine is not mentioned).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of hepatotoxicity of psychotropic drugs; guanfacine is not discussed).
- O'Donnell JM, Bies RR, Shelton RC. Pharmacotherapy of depression and anxiety disorders. In, Brunton LL, Halil-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 267-78.(Textbook of pharmacology and therapeutics).
- Jerie P. Long-term evaluations of therapeutic efficacy and safety of guanfacine. Am J Cardiol. 1986;57:55E–59E. [PubMed: 3513532](Among 580 adults with hypertension treated with guanfacine for up to two years, blood pressure normalized in 54-66% of patients and “no…biochemical alterations that could be attributed to guanfacine occurred”).
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AF, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–74. [PubMed: 11431228](Among 34 children with ADHD and tic disorders treated with guanfacine or placebo for 8 weeks, ADHD scores improved more with guanfacine and no serious side effects or “alterations in laboratory test results” occurred).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, et al. Drug Induced Liver Injury Network(DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to guanfacine).
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N., SPD503 Study Group. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–84. [PubMed: 18166547](Among 345 children and adolescents with ADHD treated with guanfacine [2, 3 or 4 mg daily] or placebo for 8 weeks, ADHD scores improved more in guanfacine treated subjects and common adverse events included sedation, somnolence, dizziness, fatigue, nausea, drug mouth and abdominal pain; no mention of ALT levels or hepatotoxicity).
- Sallee FR, Lyne A, Wigal T, McGough JJ. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:215–26. [PubMed: 19519256](Among 259 children with ADHD treated with guanfacine at varying doses for up to 24 months, the most common adverse events were somnolence and headache; no mention of ALT elevations or hepatotoxicity).
- Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J., SPD503 STUDY GROUP. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155–65. [PubMed: 19106767](Among 321 children [ages 6-17] with ADHD treated with guanfacine [1, 2, 3 or 4 mg daily] or placebo for 9 weeks, improvements in ADHD rating scores were greater with guanfacine while side effects of somnolence, headache and fatigue were more frequent, although “no clinically meaningful changes in laboratory assessments were observed for any of the study subjects”).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, with 2 agents used for ADHD among the top 41 causes; methylphenidate [11th, 96 cases] and atomoxetine [14th, 64 cases]; guanfacine was not mentioned).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury; psychotropic agents accounted for 4 cases, including quetiapine, nefazodone, fluoxetine and venlafaxine, but none were attributed to guanfacine).
- Connor DF, Findling RL, Kollins SH, Sallee F, López FA, Lyne A, Tremblay G. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24:755–68. [PubMed: 20806988](Among 217 children with ADHD treated with guanfacine or placebo for 9 weeks, adverse events that were more frequent with guanfacine included somnolence [51% vs 5%], headache [22% vs 18%], sedation [13% vs 1%], abdominal pain [12% vs 3%] and fatigue [11% vs 5%]; no mention of laboratory study results).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N. Drug-induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with suspected drug induced liver injury, 3 were attributed to atomoxetine and one to methylphenidate, but none to guanfacine).
- Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, Lyne A, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:74–85. [PubMed: 22176941](Among 461 children or adolescents with ADHD with suboptimal response to psychostimulants, those treated with guanfacine had greater improvements in ADHD rating scores than placebo controls, and there were no treatment related serious adverse events and no mention of ALT elevations or hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2-year period, but none were attributed to guanfacine or other drugs used to treat ADHD).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to guanfacine or other ADHD agents).
- Hervas A, Huss M, Johnson M, McNicholas F, van Stralen J, Sreckovic S, Lyne A, et al. Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, phase III trial. Eur Neuropsychopharmacol. 2014;24:1861–72. [PubMed: 25453486](Among 272 children or adolescents with ADHD treated for 4-7 weeks, improvements in ADHD rating scores were greater for guanfacine than atomoxetine and placebo as were adverse event rates, including somnolence, headache and fatigue, which were rarely severe; no mention of ALT elevations or hepatotoxicity).
- Wilens TE, Robertson B, Sikirica V, Harper L, Young JL, Bloomfield R, Lyne A, et al. A randomized, placebo-controlled trial of guanfacine extended release in adolescents with attention-deficit/ hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:916–25. [PubMed: 26506582](Among 314 adolescents with ADHD treated with guanfacine or placebo for 13 weeks, improvements in ADHD rating scores were greater for guanfacine and adverse events included somnolence [44% vs 21%], headache [27% v 18%] and fatigue [22% vs 12%], and “there were no clinically meaningful differences” in “clinical chemistry … analyses”).
- Drugs for ADHD. Med Lett Drugs Ther. 2015;57(1464):37–40. [PubMed: 25758544](Concise review of the mechanism of action, clinical efficacy, safety and costs of drugs approved for use in ADHD; mentions that an extended release form of guanfacine has been approved for use in ADHD and mentions adverse reactions of somnolence, bradycardia, hypotension and syncope, but does not mention ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 4 [0.5%] were attributed to atomoxetine but none to guanfacine).
- Butterfield ME, Saal J, Young B, Young JL. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: A double blind, placebo-controlled study. Psychiatry Res. 2016;236:136–41. [PubMed: 26730446](Among 26 adults with ADHD and a suboptimal response to conventional stimulants treated with guanfacine [1 to 6 mg] or placebo daily for 10 weeks, improvements in ADHD rating scores were similar in all groups as were adverse event rates; no mention of ALT elevations or hepatotoxicity and no patient stopped therapy for side effects).
- Padilha SCOS, Virtuoso S, Tonin FS, Borba HHL, Pontarolo R. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry. 2018;27:1335–45. [PubMed: 29460165](Systematic review of efficacy and safety of drugs for ADHD based upon 48 trials [4169 participants]; makes no mention of ALT elevations or hepatotoxicity of any of the agents studied, including guanfacine).
- Cortese S, Adamo N, Mohr-Jensen C, Hayes AJ, Bhatti S, Carucci S, Del Giovane C, et al. European ADHD Guidelines Group (EAGG). Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open. 2017;7:e013967. [PMC free article: PMC5253538] [PubMed: 28073796](Review of the pharmacological therapy of ADHD with discussion of amphetamines, methylphenidate, atomoxetine, guanfacine and clonidine; no mention of ALT elevations during therapy or hepatotoxicity).
- Huss M, Dirks B, Gu J, Robertson B, Newcorn JH, Ramos-Quiroga JA. Long-term safety and efficacy of guanfacine extended release in children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2018;27:1283–1294. [PubMed: 29442229](Among 215 children and adolescents with ADHD treated with dose-optimized guanfacine [1-7 mg/day] for up to 2 years, common adverse events were somnolence [36%], headache [29%], fatigue [20%] and dizziness [9%], most being transient, mild-to-moderate occurring early during dosing; there were no deaths and no serious hepatic adverse events or discontinuations for liver injury or ALT elevations).
- Schoretsanitis G, de Leon J, Eap CB, Kane JM, Paulzen M. Clinically significant drug-drug interactions with agents for attention-deficit/hyperactivity disorder. CNS Drugs. 2019;33:1201–1222. [PubMed: 31776871](Review of drug-drug interactions of agents used to treat ADHD mentions that guanfacine is metabolized largely by CYP 3A4 and levels are reduced by CYP 3A4 inducers [rifampin, phenobarbital] requiring dose increases [doubling], and are increased by CYP 3A4 inhibitors [ketoconazole] theoretically requiring halving of the dosage).
- Drugs for ADHD. Med Lett Drugs Ther. 2020;62(1590):9–15. [PubMed: 31999670](Concise review of mechanism of action, clinical efficacy, safety and cots of drugs for ADHD mentions that guanfacine is a nonstimulatory agent that is generally well tolerated; no mention of ALT elevations or hepatotoxicity).
- Iwanami A, Saito K, Fujiwara M, Okutsu D, Ichikawa H. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: Results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81:19m12979. [PubMed: 32297719](Among 201 Japanese adults with ADHD treated with guanfacine or placebo for 10 weeks, ADHD rating scales improved more with guanfacine than placebo [-11.6 vs -7.3]; while adverse events were more frequent [81% vs 62%], they were usually mild-to-moderate and there were no deaths and no serious hepatic adverse events).
- Iwanami A, Saito K, Fujiwara M, Okutsu D, Ichikawa H. Safety and efficacy of guanfacine extended-release in adults with attention-deficit/hyperactivity disorder: an open-label, long-term, phase 3 extension study. BMC Psychiatry. 2020;20:485. [PMC free article: PMC7531113] [PubMed: 33008345](Among 150 Japanese adults with ADHD enrolled in a 50 week open label extension study of guanfacine [maintenance dose 4-6 mg daily], there were no deaths, no serious hepatic adverse events, and “no clinically relevant changes in … clinical laboratory tests”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Evaluating Guanfacine Hydrochloride in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Adult Patients: Design, Development and Place in Therapy.[Drug Des Devel Ther. 2021]Review Evaluating Guanfacine Hydrochloride in the Treatment of Attention Deficit Hyperactivity Disorder (ADHD) in Adult Patients: Design, Development and Place in Therapy.Ota T, Yamamuro K, Okazaki K, Kishimoto T. Drug Des Devel Ther. 2021; 15:1965-1969. Epub 2021 May 11.
- Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration.[J Am Acad Child Adolesc Psychi...]Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration.Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, Rubin J. J Am Acad Child Adolesc Psychiatry. 2013 Sep; 52(9):921-30. Epub 2013 Aug 1.
- Stimulation of postsynapse adrenergic α2A receptor improves attention/cognition performance in an animal model of attention deficit hyperactivity disorder.[Behav Brain Res. 2014]Stimulation of postsynapse adrenergic α2A receptor improves attention/cognition performance in an animal model of attention deficit hyperactivity disorder.Kawaura K, Karasawa J, Chaki S, Hikichi H. Behav Brain Res. 2014 Aug 15; 270:349-56. Epub 2014 May 29.
- Review Guanfacine extended release: a novel treatment for attention-deficit/hyperactivity disorder in children and adolescents.[Clin Ther. 2013]Review Guanfacine extended release: a novel treatment for attention-deficit/hyperactivity disorder in children and adolescents.Faraone SV, McBurnett K, Sallee FR, Steeber J, López FA. Clin Ther. 2013 Nov; 35(11):1778-93. Epub 2013 Oct 16.
- Review Guanfacine for the treatment of attention deficit hyperactivity disorder in children and adolescents.[Expert Rev Neurother. 2015]Review Guanfacine for the treatment of attention deficit hyperactivity disorder in children and adolescents.Rizzo R, Martino D. Expert Rev Neurother. 2015 Apr; 15(4):347-54.
- Guanfacine - LiverToxGuanfacine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...