NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Gold salts, including auranofin and gold sodium thiomalate, have been used for the therapy of rheumatoid arthritis for many decades, but have recently been replaced by more modern disease-modifying antirheumatic drugs. Parenteral gold therapy (chrysotherapy) can cause acute, clinically apparent hepatitis that is usually cholestatic and self-limited in nature, but can be severe and even fatal. In contrast, oral gold preparations (such as auranofin) are associated with a low rate of serum enzyme elevations during treatment, but have not been convincingly linked to clinically apparent liver injury.
Background
Gold given as a conjugate with thioglucose, thiosulphate or thiomalate salt has disease modifying activity in rheumatoid arthritis and can induce remission in up to 50% of patients. The mechanism of action is not well defined, but gold has clear antiinflammatory activity and lowers serum immunoglobulin levels. The use of gold as an antiinflammatory agent arose out of observations by Robert Koch who reported that gold salts had in vitro activity against mycobacterium tuberculosis. Trials of gold therapy in tuberculosis were unsuccessful, but clinically apparent antiinflammatory activity was found during treatment of patients with discoid lupus erythematosus, which at that time was believed to be due to tuberculosis. Chrysotherapy was introduced in the treatment of rheumatoid arthritis in the 1930s and it became a common approach to management of severe rheumatoid arthritis until the 1960s, when it was gradually replaced by more modern forms of disease modifying antirheumatic drugs (DMARDs) such as methotrexate, leflunomide, d-penicillamine, hydroxychloroquine, sulfasalazine, enteracept and imfliximab. Previous formulations of gold salts included a parenteral form as gold sodium thiomalate which was available as 50 mg/mL under brand names including Aurolate and Myochrysine (now no longer generally available in the United States). The recommended dose was 10 mg intramuscularly once weekly, gradually increasing to a maintenance dose of 25 to 50 mg weekly or every other week. An oral form of gold, audafolin (aw ran' o fin), continues to be available as capsules of 3 mg under the brand name of Ridaura, the recommended dose of which is 3 to 9 mg daily. Therapy with gold salts is often limited by toxicity, some of which is cumulative. The effect may take weeks to months to occur, but therapy should be discontinued after an accumulative dose of 1 gram if beneficial results are not achieved. Common side effects include abdominal discomfort, nausea, pruritus, skin rash, urticaria and proteinuria. Fatal instances of hypersensitivity reactions, renal dysfunction and bone marrow suppression have been reported.
Hepatotoxicity
Therapy with parenterally administered gold salts was linked with instances of acute liver injury shortly after being introduced into therapy in the 1930s. The nature of the liver injury was not well defined. The more modern formulations of gold appear to have a lower overall incidence of toxicity, including hepatotoxicity. Mild elevations in serum aminotransferase levels are not infrequent during therapy with gold, but clinically apparent liver injury is rare and appears to be idiosyncratic in nature and has been reported only with parenteral gold therapy. The onset of injury is within 1 to 8 weeks of starting therapy and presents with fever and rash, followed by malaise, nausea, right upper quadrant pain, dark urine and jaundice. Pruritus can be prominent and the typical pattern of serum enzyme elevations is cholestatic or mixed. Recovery may be delayed but is usually complete within 6 to 8 weeks. Transient thrombocytopenia and anemia can occur, particularly in children. Autoantibody formation is not typical, although patients with rheumatoid arthritis are prone to have preexisting autoantibodies. A less frequent pattern of injury from gold therapy is marked by acute hepatic necrosis, usually arising within 1 to 4 weeks of starting therapy with marked elevations in serum aminotransferase levels (20 to 100 fold) and minimal increases in alkaline phosphatase. These cases of acute hepatocellular injury may be associated with high doses of parenteral gold salts and can be severe and have resulted in fatalities. A striking phenomenon is that symptoms of arthritis frequently improve during acute hepatic injury whether due to gold therapy or viral hepatitis. The improvements, however, are transient and of no lasting benefit.
Likelihood score (parenteral gold preparations): A (well known cause of clinically apparent liver injury).
Oral gold therapy has been associated with transient, mild elevations in serum aminotransferase levels, but there have been no convincing cases of clinically significant liver injury linked to oral preparations. Thus, idiosyncratic acute liver injury due to auranofin must be very rare, if it occurs at all.
Likelihood score (oral gold preparations): E* (unlikely although suspected cause of clinically apparent liver injury).
Mechanism of Injury
The typical acute liver injury from parenteral gold therapy appears to be related to hypersensitivity and is often accompanied by other allergic manifestations such as fever, rash, lymphadenopathy and eosinophilia. Cases of short latency onset, acute hepatic necrosis due to injections of gold more closely resemble direct, acute toxicity and have been reported after acute intentional or unintentional overdose of gold salts.
Outcome and Management
Most cases of jaundice from parenteral gold therapy are self-limited, although recovery may be prolonged. There is little evidence that chelation improves outcome or speeds recovery, although it is often used with acute overdose of a gold containing compound. Rare instances of acute hepatic necrosis soon after initiation of gold therapy have been reported, some of which have been fatal. Rechallenge after clinically apparent hepatotoxicity can lead to severe recurrence and should be avoided. In situations of mild serum aminotransferase elevations during gold therapy, reinstitution of treatment without recurrent elevations is typical.
Drug Class: Antirheumatic Agents
CASE REPORTS
Case 1. Acute cholestatic hepatitis due to gold therapy in a child.
[Modified from: Ghisolfi J, Rumeau JL, Rolland M, Dalous A. [Cholestatic syndrome following massive chrysotherapy]. Arch Fr Pediatr 1972; 29: 1097-106. French. PubMed Citation]
A 10 year old boy with suspected juvenile idiopathic (rheumatoid) arthritis was started on parenteral gold therapy in a dose of 40 mg daily for eight days (in error, as it was meant to be given twice weekly) and developed fever, rash and headaches by day nine, followed by severe nausea and vomiting leading to electrolyte imbalance and dehydration. Three days after stopping the gold injections, he was found to be jaundiced with pruritus, dark urine and light stools. The serum bilirubin was 14.6 mg/dL, alkaline phosphatase 630 U/L and ALT 130 U/L (Table). His fever and rash persisted for a week, but then improved rapidly with prednisone therapy. He remained jaundiced for a month with symptoms of pruritus and prominent elevations in serum alkaline phosphatase. A liver biopsy showed intrahepatic cholestasis. Electron microscopy demonstrated scattered gold particles in lysosomes of Kupffer cells and hepatocytes. Over the following 4 weeks the jaundice cleared and, by 6 weeks, laboratory abnormalities had returned to normal. His arthritis was managed with indomethacin.
Key Points
| Medication: | Aurothiopropanol sulfonate (total dose of 200 mg over 8 days) |
| Pattern: | Cholestatic (R=~1.3) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 9 days to fever and rash, 11 days to jaundice |
| Recovery: | 6 weeks |
| Other medications: | Indomethacin |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 14 days | 5 days | 130 | 630 | 14.6 | Fever and rash |
| 17 days | 8 days | 480 | 960 | 9.6 | |
| 19 days | 10 days | 480 | 9.8 | ||
| 26 days | 17 days | 370 | 1240 | 8.7 | Prednisone started |
| 35 days | 24 days | 400 | 1350 | 8.2 | |
| 6 weeks | 5 weeks | 240 | 1150 | 7.9 | |
| 7 weeks | 6 weeks | 83 | 620 | 5.7 | |
| 8 weeks | 7 weeks | 77 | 570 | 28 | |
| 12 weeks | 11 weeks | 35 | 400 | 1.2 | |
| Normal Values | <40 | <1.2 | |||
Comment
A cholestatic hepatitis in a child arising within a few days of stopping an 8 day course of gold therapy. The dose of gold was excessive and was meant to be given twice weekly rather than daily. Nevertheless, the pattern of injury was typical of a hypersensitivity reaction with fever and rash (DRESS syndrome, although no mention made of eosinophil counts). While the rash and fever appeared to respond to prednisone therapy, the jaundice was more persistent. Chelation therapy was not used.
Case 2. Acute cholestatic hepatitis due to gold therapy in an adult.
[Modified from: Pessayre D, Feldmann G, Degott C, Ulmann A, Roger W, Erlinger S, Benhamou JP. Gold salt-induced cholestasis. Digestion 1979; 19: 56-64. PubMed Citation]
A 66 year old woman with suspected rheumatoid arthritis was treated with parenteral injections of gold salts twice weekly for 4 weeks and developed nausea and vomiting followed by rash, jaundice and pruritus. She had no history of liver disease and did not drink alcohol. No mention was made of other medications being taken. On presentation, the serum bilirubin was 32.5 mg/dL, ALT 550 U/L, and alkaline phosphatase 72 Bodansky units (16 fold elevated) (Table). Her jaundice worsened despite stopping gold therapy. ERCP showed a normal biliary tract, and liver biopsy showed marked intrahepatic cholestasis. The rash became exfoliative and persisted for 2 months, while jaundice lasted for 3 months. More extensive evaluation of her joint complaints demonstrated Waldenstrom’s macroglobulinemia with involvement of the knee joint. When seen in follow up 9 months later for recurrence of arthritis, she was anicteric and asymptomatic of liver disease, but had mild elevations in alkaline phosphatase.
Key Points
| Medication: | Aurothiopropanol sulfonate (total dose of 200 mg over 4 weeks) |
| Pattern: | Cholestatic (R=0.8) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 4 weeks |
| Recovery: | 6 weeks |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P* (BU/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| 5 weeks | 1 week | 30 | 12.5 | ||
| 6 weeks | 2 weeks | 550 | 72 | 32.5 | Admission |
| 7 weeks | 3 weeks | 360 | 55 | 48.0 | |
| 8 weeks | 4 weeks | 180 | 52 | 40.0 | |
| 9 weeks | 5 weeks | 100 | 30 | 13.0 | |
| 10 weeks | 6 weeks | 90 | 26 | 2.0 | Discharged |
| 5 months | 4 months | 50 | 8 | <1.0 | |
| 8 months | 7 months | 40 | 6 | <1.0 | Follow up |
| Normal Values | <45 | <5 | <1.2 | ||
* Times and laboratory values are estimated from Figure 3. BU= Bodansky units
Comment
A cholestatic hepatitis in an adult with rheumatoid arthritis arising after 4 weeks of therapy with twice-weekly injections of gold. Rash and eosinophilia were prominent, but fever was not mentioned. The cholestasis was prolonged, lasting for almost three months and with minor elevations in alkaline phosphatase still present months later when she was seen for an exacerbation of her rheumatoid arthritis. The residual alkaline phosphatase elevation was attributed to involvement of the liver by Waldenstrom’s macroglobulinemia.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Auranofin - Generic (Audafolin), Ridaura®
Gold Sodium Thiomalate - Generic, Aurolate®, Myochrysine®
DRUG CLASS
Antirheumatic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Auranofin | 34031-32-8 | C20-H34-Au-O9-P-S |
 |
| Gold Sodium Thiomalate | 39377-38-3 | C4-H5-Au-O4-S.H2-O.2Na |
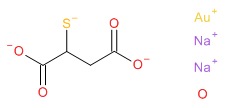 |
ANNOTATED BIBLIOGRAPHY
References updated: 02 August 2017
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-54.(Expert review of hepatotoxicity published in 1999; in early era chrysotherapy, reports of liver injury were common, but with more modern forms of gold, hepatotoxicity is rare; dermatitis, renal injury and myelotoxicity are more frequent side effects).
- Lewis JH. Stine JG. Gold salts. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 388.(Review of liver injury due to gold; cholestatic injury is the characteristic lesion, probably immunologically mediated).
- Burke A, Smyth E, FitzGerald GA. Analgesic-antipyretic and antiinflammatory agents: pharmacotherapy of gout. In, Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill, 2006, pp. 671-715.(Textbook of pharmacology and therapeutics; gold is now rarely used and has been replaced by more modern disease modifying antirheumatic drugs).
- Drive JR, Weller JN. Untoward results from the use of gold compounds. Results of a fatal case. Arch Derm Syph 1931; 23: 87-109. Not in PubMed.(23 page case report and review of the literature of adverse effects of gold therapy; 41 year old with lupus erythematosis developed fever and rash after 2nd injection of gold thiosulphate followed by confusion, jaundice, diffuse bleeding, coma and death).
- Hartfall SJ, Garland HG. Further observations on the gold treatment of rheumatoid arthritis. Lancet 1936; 1: 1459-63. Not in PubMed.(Among 300 patients with rheumatoid arthritis treated with gold injections [provided by "Messrs Schering and Bayer"], 37% had toxic reactions, including 11 [1%] with jaundice).
- Hartfall SJ, Garland HG, Goldie W. Gold treatment of arthritis. Lancet 1937; 2: 838-42. Not in PubMed.(Among 900 cases of rheumatic conditions [rheumatoid and osteoarthritis, ankylosing spondylitis and others] treated with gold, adverse events occurred in 42% and were severe in 6%; jaundice in 85 [9.4%], which was severe in 13 [1.4%] and fatal in 2; often during first course, not recurring with retreatment; some cases may have represented coincidental acute viral hepatitis).
- Hartung EF. The treatment of rheumatoid arthritis including gold salts therapy. Bull N Y Acad Med 1943; 19: 693-703. [PMC free article: PMC1934041] [PubMed: 19312340](Review of role of gold in management of rheumatoid arthritis; given parenterally in courses of up to 1-2 grams cumulative, with 2-3 months between courses; no benefit if first course is not effective; remissions in 54% of patients; lists toxic hepatitis as a rare side effect).
- Hartung EF. Toxic hepatitis during gold salt therapy: its effect on the course of rheumatoid arthritis. Med Clin North Am 1946; 30: 553-61. Not in PubMed. [PubMed: 20983788](Among 800 patients with rheumatoid arthritis treated with gold, only 2 developed jaundice; symptoms of arthritis improved in half of patients who developed liver injury during gold therapy, but the improvement was only partial and transient only).
- Schwartz S, Blain HR, Geiger HB, Hartung EF. Investigation of use of aurothioglycanide(Lauron) in rheumatoid arthritis: preliminary report of toxicity and therapeutic effects of a fine suspension. J Am Med Assoc 1954; 154: 1263-5. [PubMed: 13151835](Among 56 patients with rheumatoid arthritis treated with aurothioglycanide injections, 14% developed rash but none were severe, 1 developed thrombocytopenia, no mention of liver injury).
- Moeschlin S, Siegenthaler P. [Cholestatic hepatitis caused by gold; analogy to chlorpromazine and androgen icterus.] Helv Med Acta 1960; 27: 707-15. [PubMed: 13771657](47 year old woman with rheumatoid arthritis developed nausea and jaundice 3 weeks after starting gold therapy with prolonged jaundice and pruritus [bilirubin 7.5 rising to 24 mg/dL, Alk P 2-3 times ULN, ALT 134 U/L, 2% eosinophils on corticosteroids], laparotomy and liver biopsy showing intrahepatic cholestasis).
- Empire Rheumatism Council Research Sub-Committee. Gold therapy in rheumatoid arthritis. Final report of a multicenter controlled trial. Ann Rheum Dis 1961; 20: 315-34. Not in PubMed. [PMC free article: PMC1007231] [PubMed: 18623862](In a controlled trial of aurothiomalate in 185 patients with rheumatoid arthritis with 2 years of follow up, 35% had side effects; usually rash [20%], purpura, proteinuria, fever and gastrointestinal upset; no mention of liver injury).
- Wiontzek H, Schmidt J. [Allergic cholestatic hepatosis following gold treatment of a seronegative, chronic progressive polyarthritis]. Z Rheumaforsch 1970; 9: 46-8. German. [PubMed: 5420462](50 year old woman developed an allergic reaction after 43 days of gold therapy with eosinophilia [35%] and minor elevations of Alk P [170 U/L] and ALT [41 U/L] without jaundice, resolving in 2 weeks).
- Kornreich H, Malouf NN, Hanson V. Acute hepatic dysfunction in juvenile rheumatoid arthritis. J Pediatr 1971; 79: 27-35. [PubMed: 5314582](7 children with juvenile idiopathic [rheumatoid] arthritis developed acute hepatic injury from various causes; ages 2-8 years, 5 with jaundice, all had transient remission during peak of injury regardless of cause with decrease in ESR and rheumatoid factor; 3 cases were receiving gold therapy and several others were on high doses of aspirin or indomethacin).
- Ghisolfi J, Rumeau JL, Rolland M, Dalous A. [Cholestatic syndrome following massive chrysotherapy]. Arch Fr Pediatr 1972; 29: 1097-106. French. [PubMed: 4662281](10 year old boy given 40 mg of gold daily for 8 days, developed rash, vomiting, fever and jaundice [bilirubin 14.6 mg/dL, Alk P 630 rising to 1350 U/L, ALT 150 U/L], resolving within 6 weeks of stopping; gold identified in liver biopsy in lysosomes of Kupffer cells and hepatocytes: Case 1).
- Gigante D. [Collateral effects of gold salts in the therapy of rheumatoid arthritis]. Clin Ter 1972; 60: 321-32. Italian. [PubMed: 5023985]
- Iuel J. [Deaths from gold therapy in Denmark during 1960-64]. Ugeskr Laeger 1973; 135: 1639-42. Danish. [PubMed: 4271249](Among 10 reports of fatalities attributed to gold, 2 were due to liver injury: 82 year old woman with mixed connective tissue disease had onset of fever and jaundice 3 days after initial injection and died 15 days later; 14 year old boy with juvenile idiopathic [rheumatoid] arthritis who developed fever and jaundice 2 days after the second injection and died 7 days later).
- Schenker S, Olson KN, Dunn D, Breen KJ, Combes B. Intrahepatic cholestasis due to therapy of rheumatoid arthritis. Gastroenterology 1973; 64: 622-9. [PubMed: 4633722](Two case reports; 66 year old man with rheumatoid arthritis developed jaundice after third weekly injection of gold [bilirubin 7.6 mg/dL, ALT 73 U/L, Alk P 10 times ULN], with complete resolution over next 2 months; 34 year old woman with rheumatoid arthritis developed rash and jaundice after fourth weekly injection of gold [bilirubin 12 rising to 45 mg/dL, AST 83 U/L, Alk P 5 times ULN], with prolonged jaundice and pruritus for 6 months and abnormal Alk P 2 years later).
- Cuthbert MF. Adverse reactions to non-steroidal antirheumatic drugs. Curr Med Res Opin 1974; 2: 600-10. [PubMed: 4452298](Summary of adverse reaction reports on rheumatologic agents from UK; 102 reports on gold over 10 year period, 32 being fatal; mostly blood disorders, no mention of hepatotoxicity).
- Favreau M, Tannenbaum H, Lough J. Hepatic toxicity associated with gold therapy. Ann Intern Med 1977; 87: 717-9. [PubMed: 412451](Three cases of liver injury due to gold therapy in patients with rheumatoid arthritis; women, ages 50-59 years, onset of jaundice 20-40 days after starting gold injections, peak bilirubin 7.1-11.7 mg/dL, AST 62-230 U/L, Alk P 5-7 times ULN, fever in 1, eosinophilia in 2, slow but complete recovery in all).
- Prichanond S, Skosey JL. Gold and liver biopsy. Ann Intern Med 1978; 88: 579. [PubMed: 416737](44 year old woman with rheumatoid arthritis developed large lymph nodes, hepatomegaly and pulmonary infiltrates after 135 mg of gold injections with eosinophila, but minimal to no elevation in AST, Alk P or bilirubin, slowly resolved and liver biopsy was unrevealing).
- Fossati C. [Side-effects and toxic effects of gold salts]. Clin Ter 1979; 91: 407-20. Italian. [PubMed: 161525]
- Ghishan FK, LaBrecque DR,Younoszai K. Intrahepatic cholestasis after gold therapy in juvenile rheumatoid arthritis. J Pediatr 1978; 93: 1042-3. [PubMed: 102752](Child with juvenile idiopathic [rheumatoid] arthritis developed fever, rash and abdominal pain 48 hours after second injection of gold [bilirubin 13 mg/dL, ALT 120 U/L, Alk P 575 U/L], resolving within 8 weeks of stopping).
- Panayi GS, Wooley P, Batchelor JR. Genetic basis of rheumatoid disease: HLA-antigens, disease manifestations, and toxic reactions to drugs. Brit Med J 1978; 8: 1326-8. [PMC free article: PMC1608410] [PubMed: 719380](Among 95 patients with rheumatoid arthritis, 56% had DRW4 [vs 33% of controls], minor associations of toxicity to gold or penicillamine in subjects with DRW2 or 3; no mention of hepatotoxicity).
- Pessayre D, Feldmann G, Degott C, Ulmann A, Roger W, Erlinger S, Benhamou JP. Gold salt-induced cholestasis. Digestion 1979; 19: 56-64. [PubMed: 156659](66 year old woman with rheumatoid arthritis developed jaundice 4 weeks after starting parenteral gold therapy [bilirubin 32.5 mg/dL, ALT 550 U/L, Alk P 14 times ULN, eosinophils 1,400/µL], slow resolution and mild Alk P abnormalities 9 months later: Case 2).
- Griffin AJ. Cholestatic hepatitis induced by gold. Rheumatol Rehabil 1979; 18: 174-6. [PubMed: 115078](55 year old man with rheumatoid arthritis developed jaundice 14 days after starting gold therapy [peak bilirubin 4.9 mg/dL, AST 82 U/L, Alk P 918 U/L], worsening for 1 week and then resolving).
- Alcalay M, Touchard G, Patte F, Babin P, Reboux JF, Thomas P, Patte D, et al. [Simultaneous nephropathy, lung disease and liver disease caused by gold salts, with an ultrastructural study of pulmonary and renal lesions]. Rev Rhum Mal Osteoartic 1979; 46: 491-8. French. [PubMed: 504952]
- Kean WF, Anastassiades TP. Long term chrysotherapy: incidence of toxicity and efficacy during sequential time periods. Arthritis Rheum 1979; 22: 495-501. [PubMed: 156026](Among 94 patients with rheumatoid arthritis treated with gold at a single clinic, 70% had clinical benefit, 42 had to be withdrawn because of toxicity: rash in 45%, mouth ulcers in 20%, proteinuria 19%; 2 instances of jaundice after 85 and 75 mg, resolving in 6 weeks and tolerated restarting).
- Wilson DB, Korn JH. Acute toxicity from an overdose of gold. J Rheumatol 1979; 6: 359-61. [PubMed: 114637](33 year old woman with rheumatoid arthritis treated with gold for 4 years, received single dose of 500 mg by error, immediately developing headache, flushing and nausea with transient thrombocytopenia [platelet nadir: 83,000/µL] and mild serum enzyme elevations [ALT 70 U/L], resolving in 4 days).
- Gibbons RB. Complications of chrysotherapy: a review of recent studies. Arch Intern Med. 1979; 139: 343-6. [PubMed: 371565](Review of side effects of gold therapy; hepatic injury is rare but well defined, usually cholestatic and self-limited, but recovery may be slow).
- Davis P. Undesirable effects of gold salts. J Rheumatol Suppl 1979; 5: 18-24. [PubMed: 290813](Review of major complications of gold therapy including myelosuppression, nephrotic syndrome, dermatitis; under miscellaneous, cholestatic hepatitis is mentioned; chelation therapy of uncertain benefit).
- Chalès G, Grosbois B, Pawlotsky Y, Louboutin JY, Gueguen A, Estable P. [Neurological and hepatic complications in chrysotherapy (author's transl)]. Sem Hop 1980; 56: 1787-92. French. [PubMed: 6256879]
- Bene E, Megyeri A, Györi J. [Gold therapy in chronic polyarthritis. Differentiation of allergic and toxic side effects]. Z Rheumatol 1980; 39: 109-16. German. [PubMed: 6448525]
- Charhon S, Rouillat M, Bouvier M. [Hepatitis caused by gold therapy: a case]. Rev Rhum Mal Osteoartic 1980; 47: 205. French. [PubMed: 7384728](67 year old woman with rheumatoid arthritis developed rash after two gold injections followed by fever and jaundice [bilirubin 4.5 mg/dL, ALT 180 U/L, Alk P 756 U/L], resolving within 6 weeks of stopping).
- Wooley PM, Griffin J, Panayi GS, Batchelor JR, Welsh ICI, Gibson TJ. HLA-DR antigens and toxic reactions to sodium-aurothiomalate and D-penicillamine in patients with rheumatoid arthritis. N Engl J Med 1980; 303: 300-2. [PubMed: 6770269](HLA testing on another 91 patients with rheumatoid arthritis found DRw3/HLA B8 in 14 of 15 patients who developed proteinuria on gold or penicillamine therapy; DRw4 in 54% of patients).
- Frigerio G, Terruzzi V, Butti GC, Minoli G, Rossini A. [Toxic cholestatic hepatitis secondary to chrysotherapy]. Minerva Med 1981; 72: 1941-4. Italian. [PubMed: 7254640](57 year old woman with rheumatoid arthritis developed jaundice shortly after fourth weekly injection of gold [bilirubin 3.6 mg/dL, ALT 340 U/L , Alk P 120 U/L, eosinophils 1%], biopsy showing intrahepatic cholestasis, resolving in 6 weeks).
- Le Bodic MF, Degott C, Barrier J, Chekroun G, Gaillard F, Couprie F, Mussini-Montpellier J. [Anatomo-pathologic aspects of gold-salt induced cholestasis. Report of two cases with ultrastructural study (author's transl)]. Arch Anat Cytol Pathol 1981; 29: 98-104. French. [PubMed: 7235755](Two cases: 48 year old man with rheumatoid arthritis developed fever and malaise after first two injections of gold and jaundice after the third [bilirubin 2.7 mg/dL, ALT 75 U/L, Alk P 165 U/L, eosinophils 1,078/µL], with rapid and complete recovery; 66 year old woman with rheumatoid arthritis developed rash and jaundice 1 week after 4 week course of gold [bilirubin 32.5 mg/dL, ALT 550 U/L, Alk P 12 times ULN, eosinophils 1,400/µL], recovery in 3 months, but Alk P still elevated 10 months later).
- Howrie DL, Gartner JC Jr. Gold-induced hepatotoxicity: case report and review of the literature. J Rheumatol 1982; 9: 727-9. [PubMed: 6217328](3 year old girl with juvenile idiopathic [rheumatoid] arthritis developed rash, fever, lymphadenopathy and jaundice after 2nd injection of gold sodium thiomalate [bilirubin 4.0 mg/dL, AST 240 U/L, platelets 87,000/µL], rapid recovery).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol 1982; 17: 205-11. [PubMed: 6982502](Among 572 reports of drug induced liver injury from Denmark between 1968 and 78, 2% were attributed to gold therapy).
- Korneichuk AV, Kushnir LV. [Case of drug(krizanol) hepatitis]. Vrach Delo 1982; 2: 41-2. Russian. [PubMed: 7064450]
- Sanchis Closa A, Caballeria Rovira E, Arago Lopez JV, Sune Gispert S. [Submassive hepatic necrosis due to treatment with gold salts]. Gastroenterol Hepatol 1983; 6: 468-70. Spanish. Not in PubMed.(32 year old woman with rheumatoid arthritis developed jaundice 6 months after starting gold therapy with aurothioglucose [bilirubin 2.4 mg/dL, ALT 400 U/L]; therapy was continued for another 2 months when she developed exfoliative rash and purpura [bilirubin 2.8 mg/dL, ALT 175 U/L, Alk P 5 times ULN] and bone marrow failure leading to death 17 days later; postmortem liver biopsy reportedly showed massive hepatic necrosis).
- Gran JT, Husby G, Thorsby E. HLA DR antigens and gold toxicity. Ann Rheum Dis 1983; 42: 63-6. [PMC free article: PMC1001062] [PubMed: 6402993](Among 132 patients with rheumatoid arthritis treated with gold, 65% had adverse reactions, 42% rash, 11% proteinuria and 3.8% liver injury; the 16 patients with HLA DR3 had higher rates of adverse reactions including 44% with proteinuria and 13% liver reactions).
- Ward JR, Williams HJ, Egger MJ, Reading JC, Boyce E, Altz-Smith M, Samuelson CO Jr, et al. Comparison of auranofin, gold sodium thiomalate, and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum 1983; 26: 1303-15. [PubMed: 6416259](Controlled trial of oral auranofin vs parenteral gold sodium thiomalate vs placebo in 193 patients with rheumatoid arthritis; 3 patients [4%] on gold injections, but none on oral gold or placebo had ALT elevations on therapy, which fully resolved on stopping).
- Edelman J, Donnelly D, Graham N, Percy JS. Liver dysfunction associated with gold therapy for rheumatoid arthritis. J Rheumatol 1983; 10: 510-1. [PubMed: 6411922](Three cases: 65, 61 and 56 year old women with rheumatoid arthritis developed symptoms of liver injury [jaundice, nausea and pruritus] after 8, 12 and 2-3 weeks of gold sodium thiomalate therapy [bilirubin 32.8, 8.8 and 2.0 mg/dL, AST 61, 151 and 50 U/L, Alk P 355, 635 and 350 U/L], recovery in 1-3 months, but persistent Alk P elevations still present in all 3 when last seen 5-20 weeks after stopping gold).
- Lewis D, Madhok R, Capell H. Liver dysfunction associated with gold therapy for RA. J Rheumatol 1984; 11: 395-6. [PubMed: 6429329](72 year old woman with rheumatoid arthritis developed jaundice 3-4 weeks after starting gold therapy [bilirubin 7.1 mg/dL, ALT 169 U/L, Alk P 2890 U/L], resolving within 1 month).
- Rainer F. [Undesirable effects of gold therapy]. Wien Klin Wochenschr Suppl 1984; 156: 40-4. German. [PubMed: 6442056](Review of side effects of gold therapy; rare but severe complications of gold therapy are pulmonary fibrosis, aplastic anemia, thrombocytopenia, leukopenia, neuropathy, loss of hair, nail changes and cholestatic liver injury, these being "extremely uncommon or no more than anecdotal").
- Shaban MR, Golding DN, Letcher RG. Fatal intrahepatic cholestasis and interstitial lung fibrosis following gold therapy for rheumatoid arthritis. J R Soc Med 1984; 77: 960-1. [PMC free article: PMC1440227] [PubMed: 6438309](53 year old woman with rheumatoid arthritis developed fever after a second injection of gold with progressive pneumonitis and jaundice arising 2 days later [bilirubin 6.5 mg/dL, AST 198 U/L, Alk P 2080 U/L, 8% eosinophils] and death within days; autopsy showed centrolobular necrosis and cholestasis).
- Smith MD, Brooks PM. Gold compounds in rheumatic diseases-2. Med J Australia 1984; 140: 77-81. [PubMed: 6607399](Review on use of gold in rheumatoid arthritis; side effects occur in 50% of patients, skin rashes in 24%, stomatitis in 11%, proteinuria in 10%, and thrombocytopenia in 4%; pneumonitis and hepatitis from gold are rare).
- Schapira D, Nahir M, Scharf Y, Pollack S. Cholestatic jaundice induced by gold salts treatment clinical and immunological aspects--report of one case and review of the literature. J Rheumatol 1984; 11: 843-5. [PubMed: 6440983](56 year old woman with rheumatoid arthritis developed rash and jaundice 4 weeks after starting aurothioglucose [bilirubin 10.8 mg/dL, AST 95 U/L, Alk P 1525 U/L], with positive in vitro lymphocyte stimulation tests to gold).
- Jaeger A, Porte A, Sauder P, Stoeckel ME, Mantz JM. [Hepatitis secondary to a gold salt overdose]. Gastroenterol Clin Biol 1984; 8: 660-6. French. [PubMed: 6489687](51 year old woman with rheumatoid arthritis mistakenly took an overdose of aurothiopropanol sulfonate [300 mg gold] and two weeks later developed fever and jaundice [bilirubin peak 11 mg/dL, ALT 665 U/L, Alk P 2020 U/L, 1,400/µL eosinophils], resolving with 6 weeks of stopping).
- Lowthian PJ, Cleland LG, Vernon-Roberts B. Hepatotoxicity with aurothioglucose therapy. Arthritis Rheum 1984; 27: 230-2. [PubMed: 6421293](43 year old woman with rheumatoid arthritis developed lymphadenopathy followed by lethargy and dark urine, rash and eosinophilia 2 weeks after starting aurothioglucose injections [bilirubin 1.2 mg/dL, AST 166 U/L, Alk P 542 U/L], resolving within 2 months).
- Harats N, Ehrenfeld M, Shalit M, Lijovetzky G. Gold-induced granulomatous hepatitis. Isr J Med Sci 1985; 21: 753-6. [PubMed: 3932253](53 year old woman with rheumatoid arthritis developed jaundice and pruritus without rash or fever 3 weeks after starting aurothioglucose [bilirubin 4.4 mg/dL, ALT 356 U/L, Alk P 344 U/L], liver biopsy showing intrahepatic cholestasis and a few small, foamy granulomas).
- Hadchouel M, Prieur AM, Griscelli C. Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: possible relationship to drugs or infection. J Pediatr 1985; 106: 561-6. [PubMed: 3981309](7 French children with juvenile idiopathic [rheumatoid] arthritis developed sudden onset of fever, vomiting, drowsiness, with gastrointestinal hemorrhagic and liver abnormalities, with marked elevations in ALT and prothrombin time, two died, several were treated with corticosteroids, 3 had onset soon after starting parenteral gold therapy).
- Hanissian AS, Rothschild BM, Kaplan S. Gold: hepatotoxic and cholestatic reactions. Clin Rheumatol 1985; 4: 183-8. [PubMed: 3924468](Four cases of jaundice arising during gold therapy of juvenile or adult rheumatoid arthritis with few specific details; all resolved rapidly with stopping therapy).
- Hochberg MC. Auranofin or d-penicillamine in the treatment of rheumatoid arthritis. Ann Intern Med 1986; 105: 528-35. [PubMed: 3092712](Controlled trial of 12 months of auranofin vs penicillamine in 90 patients with rheumatoid arthritis; withdrawal rate was 40%; abnormal liver tests [>2 times ULN] occurred in 11% vs 7%).
- Smith MD. Hepatitis and neutropenia secondary to gold thiomalate therapy for rheumatoid arthritis. Aust N Z J Med 1986; 16: 72-4. [PubMed: 3085648](37 year old woman with rheumatoid arthritis developed jaundice after 80 mg of gold therapy [bilirubin ~19 mg/dL, AST ~230 U/L, Alk P ~150 U/L, 5% eosinophils], with recurrence and severe neutropenia upon rechallenge).
- Schapira D, Nahir AM. [Chrysotherapy induced hepatic damage]. Harefuah 1986; 110: 14-5. Hebrew. [PubMed: 3082727]
- Tozman ECS, Gottlieb NL. Adverse reactions with oral and parenteral gold preparations. Med Toxicol 1987; 2: 177-89. [PubMed: 3298922](In the US, 2 parenteral gold formulations [aurothioglucose and aurothiomalate] and one oral form [auranofin: triethylphosphine gold] are available; indications are progressive rheumatoid arthritis).
- Tebib J, Colson F, Noël E, Bouvier M. [A new case of gold-induced hepatitis during the treatment of rheumatoid polyarthritis]. Rev Rhum Mal Osteoartic 1988; 55: 705-7. French. [PubMed: 3142032]
- Farre JM, Perez T, Hautefeuille P, Tonnel F, Colombel JF, Duquesnoy B, Delcambre B. Cholestasis and pneumonitis induced by gold therapy. Clin Rheumatol 1989; 8: 538-40. [PubMed: 2612124](70 year old man with rheumatoid arthritis developed rash and fever after 3 weeks of weekly gold injections [bilirubin 10.4 mg/dL, ALT normal, Alk P 990 U/L]; biopsy showed intrahepatic cholestasis, lymphocyte stimulation tests to gold was positive, resolution within 12 weeks).
- Watkins PB, Schade R, Mills AS, Carithers RL Jr, Van Thiel DH. Fatal hepatic necrosis associated with parenteral gold therapy. Dig Dis Sci 1988; 33: 1025-9. [PubMed: 3134176](2 cases of severe hepatotoxicity from gold in patients with seronegative rheumatoid arthritis: 33 year old man developed abdominal pain, fever and jaundice 1 day after second injection of aurothioglucose [bilirubin 20.7 mg/dL, ALT 2380 U/L, Alk P 342 U/L, protime 3 sec prolonged], with ascites and progressive coma leading to liver transplantation; 23 year old man developed fatigue and jaundice 2 months after starting aurothiomalate [bilirubin 7.7 mg/dL, ALT 2760 U/L, Alk P 397 U/L, protime 6 sec prolonged], with progressive hepatic failure and death).
- González Pérez JA, Calvo Catala J, Herrera Bataller A, Hortelano Martínez E, Folgado Montesinos J, López Maldonado MD. [Mixed hepatopathy due to parenteral gold salts]. An Med Interna 1990; 7: 477-9. Spanish. [PubMed: 1983295]
- Hansen RM, Varma RR, Hanson GA. Gold induced hepatitis and pure red cell aplasia. Complete recovery after corticosteroid and N-acetylcysteine therapy. J Rheumatol 1991; 18: 1251-3. [PubMed: 1941835](54 year old man with psoriatic arthritis developed jaundice 4 weeks after starting gold sodium thiomalate injections [bilirubin peak 26 mg/dL, ALT 310 U/L, Alk P 1372 U/L], followed by pure red cell aplasia, liver injury resolving in 6 weeks and anemia in 8 weeks).
- Singh G, Fries JF, Williams CA, Zatarain E, Spitz P, Bloch DA. Toxicity profiles of disease modifying antirheumatic drugs in rheumatoid arthritis. J Rheumatol 1991; 18: 188-94. [PubMed: 1673721](Analysis of side effects of 7 agents from the ARAMIS database including 2,479 patients [582 on parenteral and 322 on oral gold] with rheumatoid arthritis reported no instances of liver abnormalities or jaundice during ~1230 patient years of exposure).
- Van Linthoudt D, Buss W, Beyner F, Ott H. [Fatal hepatic necrosis due to a treatment course of rheumatoid arthritis with gold salts]. Schweiz Med Wochenschr 1991; 121: 1099-102. French. [PubMed: 1678206]
- Fleischner GM, Morecki R, Hanaichi T, Hayashi H, Quintana N, Sternlieb I. Light- and electron-microscopical study of a case of gold salt-induced hepatotoxicity. Hepatology 1991; 14: 422-5. [PubMed: 1908434](56 year old woman with rheumatoid arthritis developed jaundice after 8 years of intermittent gold therapy [bilirubin 10.2 mg/dL, ALT 487 U/L, Alk P 99 U/L], biopsy showed submassive necrosis and inflammation but no mention of fibrosis, slow improvement and persistence of ALT elevations 10 months after stopping).
- Yamanishi Y, Takeda M, Honjo N, Aoi K, Ishibe Y, Yamana S. [Gold-induced severe cholestatic jaundice in rheumatoid arthritis patient and effect of repeated steroid pulse therapy]. Ryumachi 1992; 32: 475-82. Japanese. [PubMed: 1440084]
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med 1992; 232: 133-8. [PubMed: 1506809](Among 1188 liver adverse drug reaction reports in Denmark between 1979 and 1987, gold accounted for 19 [1.6%] overall including 2 of 52 fatal cases [4%]).
- Brass EP. Hepatic toxicity of antirheumatic drugs. Cleve Clin J Med 1993; 60: 466-72. [PubMed: 8287508](Short review of hepatotoxicity of nonsteroidals, salicylates, methotrexate, penicillamine and gold).
- Rye B, Krusinski PA. Hepatonecrosis resulting from parenteral gold therapy in pemphigus vulgaris. J Am Acad Dermatol 1993; 28: 99-101. [PubMed: 8425980](62 year old woman with pemphigus vulgaris developed jaundice 3 weeks after starting gold sodium thiomalate [bilirubin 6.7 mg/dL, ALT 1265 U/L, Alk P 308 U/L, no eosinophils], resolving in 8 weeks on dexamethasone).
- Nisar M, Winfield J. Gold induced colitis and hepatic toxicity in a patient with rheumatoid arthritis. J Rheumatol 1994; 21: 938-9. [PubMed: 8064738](40 year old woman with rheumatoid arthritis developed rash and jaundice after 4 weeks of aurothiomalate injections [bilirubin 7.5 mg/dL, AST 68 U/L, Alk P 320 U/L, eosinophils normal], with colitis leading to laparotomy and partial colectomy; cholestasis resolved within 2 months, but minor Alk P elevations persisted).
- Bendix G, Bjelle A. A 10 year follow up of parenteral gold therapy in patients with rheumatoid arthritis. Ann Rheum Dis 1996; 55: 169-76. [PMC free article: PMC1010123] [PubMed: 8712879](Long term outcome on 376 patients with rheumatoid arthritis treated with gold, high rate of discontinuation ~42% at 1, 55% at 2, 74% at 5 and 92% by 10 years; mucocutaneous side effects were most common reason for stopping therapy; one patient died of acute liver failure after 9 months of gold therapy, cause uncertain).
- Bustabad Reyes S, González García T, Trujillo Martín E, Gantes Mora M, Rodríguez-Lozano B [Toxic effects of the second dose of sodium aurothiomalate in childhood systemic chronic arthritis]. An Esp Pediatr 1997; 46: 391-3. Spanish. [PubMed: 9214235]
- Tilelli JA, Heinrichs MM. Adverse reactions to parenteral gold salts. Lancet 1997; 349: 853. [PubMed: 9121270](71 year old woman with rheumatoid arthritis on weekly aurothioglucose for 9 months, developed anaphylaxis to single injection of aurothiomalate, with subsequent cerebrovascular accident and death).
- Koryem HK, Taha KM, Ibrahim IK, Younes LK. Liver toxicity profile in gold-treated Egyptian rheumatoid arthritis patients. Int J Clin Pharmacol Res 1998; 18: 31-7. [PubMed: 9604732](40 Egyptian patients with rheumatoid arthritis treated with gold for at least 40 weeks underwent liver biopsy; 16 had fibrosis [Roenigk Grade IIIA or B], but none had "significant abnormal deviations" in ALT and AST results).
- Ben-Ami H, Pollack S, Nagachandran P, Lashevsky I, Yarnitsky D, Edoute Y. Reversible pancreatitis, hepatitis, and peripheral polyneuropathy associated with parenteral gold therapy. J Rheumatol 1999; 26: 2049-50. [PubMed: 10493691](63 year old man with rheumatoid arthritis developed abdominal pain, fever and jaundice 2-3 weeks after starting gold injections [bilirubin 7.1 rising to 25.8 mg/L, ALT 903 U/L, Alk P 298 U/L, amylase 967 U/L, no eosinophils], with appearance of polyneuropathy 2 weeks later and resolution over 4 months; lymphocyte stimulation test to gold was weakly positive).
- te Boekhorst PA, Barrera P, Laan RF, van de Putte LB. Hepatotoxicity of parenteral gold therapy in rheumatoid arthritis: a case report and review of the literature. Clin Exp Rheumatol 1999; 17: 359-62. [PubMed: 10410273](62 year old man with rheumatoid arthritis developed jaundice 3 weeks after starting gold injections [bilirubin ~5.3 mg/dL, ALT 305 U/L, Alk P 210 U/L], resolving in 6 weeks).
- van Jaarsveld CHM, Jahangier ZN, Jacobs JWG, Blaauw AAM, van Albada-Kuipers GA, ter Borg EJ, Brus HLM, et al. Toxicity of antirheumatic drugs in a randomized clinical trial of early rheumatoid arthritis. Rheumatology 2000; 39: 1374-82. [PubMed: 11136881](Controlled trial of 4 treatment strategies in 419 patients with early rheumatoid arthritis; side effects were common with ALT elevations in 5 patients on nonsteroidals only, 1 patient each on gold and hydroxychloroquine, and 20 patients on methotrexate).
- Mok MY, Ng WL, Yuen MF, Wong RW, Lau CS. Safety of disease modifying anti-rheumatic agents in rheumatoid arthritis patients with chronic viral hepatitis. Clin Exp Rheumatol 2000; 18: 363-8. [PubMed: 10895374](Among 29 Chinese patients with rheumatoid arthritis and chronic hepatitis [23 HBV; 6 HCV], ALT elevations occurred in 41% on hydroxychloroquine, 30% on methotrexate and 14% on gold vs 14% of 94 controls without viral hepatitis).
- Uhm WS, Yoo DH, Lee JH, Kim TH, Jun JB, Lee IH, Bae SC, et al. Injectable gold-induced hepatitis and neutropenia in rheumatoid arthritis. Korean J Intern Med. 2000; 15: 156-9. [PMC free article: PMC4531758] [PubMed: 10992732](62 year old woman with rheumatoid arthritis developed nausea and pruritus 2-3 weeks after starting gold therapy [bilirubin normal, ALT ~190 U/L, Alk P ~370 U/L], resolving within 4 weeks).
- Wu ML, Tsai WJ, Ger J, Deng JF, Tsay SH, Yang MH. Cholestatic hepatitis caused by acute gold potassium cyanide poisoning. J Toxicol Clin Toxicol 2001; 39: 739-43. [PubMed: 11778673](27 year old man took overdose of gold potassium cyanide and was jaundiced one day later [bilirubin 5.6 mg/dL, ALT 273 U/L, Alk P 117 U/L], resolving over next 3 weeks).
- Ciompi ML, Amoresano C, Balzarini P, Bazzichi LM, Broggini M, Buratti L, Calcagnile F, et al. [Sodium gold thiosulfate therapy: an open, viewed, multicenter trial in rheumatoid arthritis patients followed for two years]. Reumatismo 2002; 54: 251-6. Italian. [PubMed: 12404034](Among 126 patients with rheumatoid arthritis treated with gold sodium thiomalate for two years, liver test abnormalities accounted for dose discontinuations in ~2% of patients).
- Basset C, Vadrot J, Denis J, Poupon J, Zafrani ES. Prolonged cholestasis and ductopenia following gold salt therapy. Liver Int 2003; 23: 89-93. [PubMed: 12654130](49 year old woman with suspected rheumatoid arthritis developed rash and jaundice one month after starting aurothiopropanol sulphonate injections [bilirubin 7 mg/dL, ALT 5 times, and Alk P 11 times ULN], biopsy showing bile duct injury, slow resolution, ductopenia in follow up liver biopsy).
- Ujfalussy I, Koó E, Seszták M, Gergely P. Termination of disease-modifying antirheumatic drugs in rheumatoid arthritis and in psoriatic arthritis. A comparative study of 270 cases. Z Rheumatol 2003; 62: 155-60. [PubMed: 12721703](Among 206 patients treated with methotrexate, sulphasalazine or gold therapy, withdrawals due to toxicity and to elevated serum enzymes were more common in patients with psoriasis [47% and 11.5%] than rheumatoid arthritis [29% and 5.7%]).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to gold).
- Díaz J, Dávalos M, Román R, Bustíos C, Zumaeta E. [Hepatotoxicity and pancreatitis associated with gold salts: case report]. Rev Gastroenterol Peru 2004; 24: 353-6. Spanish. [PubMed: 15614305](Abstract; 37 year old woman with rheumatoid arthritis developed jaundice after 3 weeks of gold therapy).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](Survey of all cases of DILI with fatal outcome from Swedish Adverse Drug Reporting System from 1966-2002; 103 cases identified as highly probable, probable or possible; none were attributed to gold).
- Brenard R, Dumortier P, Del Natale M, Honhon B, Van Den Berghe M, Bataille C, Rickaert F, et al. Black pigments in the liver related to gold and titanium deposits. A report of four cases. Liver Int 2007; 27: 408-13. [PubMed: 17355464](Black pigment found in portal tract macrophages and Kupffer cells in liver biopsies from 4 patients with chronic cholestasis was shown to be gold in 3, two on long term gold therapy for rheumatoid arthritis and one who had no memory of receiving gold; a fourth patient had pigment in macrophages of granulomas that was shown to be titanium [bilateral knee replacements]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to gold).
- Helliwell PS, Taylor WJ; CASPAR Study Group. Treatment of psoriatic arthritis and rheumatoid arthritis with disease modifying drugs comparison of drugs and adverse reactions. J Rheumatol 2008; 35: 472-6. [PubMed: 18203324](In a large multinational community based database of patients with psoriatic and rheumatoid arthritis, gold salts were used in 7-11%).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, one was attributed to "gold salts" [Diaz 2004]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to gold salts).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Role of disease-modifying antirheumatic drugs versus cytotoxic agents in the therapy of rheumatoid arthritis.[Am J Med. 1988]Review Role of disease-modifying antirheumatic drugs versus cytotoxic agents in the therapy of rheumatoid arthritis.Ward JR. Am J Med. 1988 Oct 14; 85(4A):39-44.
- Review Comparison of the kinetics of parenteral and oral gold.[Scand J Rheumatol Suppl. 1983]Review Comparison of the kinetics of parenteral and oral gold.Gottlieb NL. Scand J Rheumatol Suppl. 1983; 51:10-4.
- Comparison of auranofin, gold sodium thiomalate, and placebo in the treatment of rheumatoid arthritis. Subsets of responses.[Am J Med. 1983]Comparison of auranofin, gold sodium thiomalate, and placebo in the treatment of rheumatoid arthritis. Subsets of responses.Ward JR, Williams HJ, Boyce E, Egger MJ, Reading JC, Samuelson CO Jr. Am J Med. 1983 Dec 30; 75(6A):133-7.
- Review [Comparison of oral and parenteral gold therapy--review of the literature].[Wien Klin Wochenschr Suppl. 1984]Review [Comparison of oral and parenteral gold therapy--review of the literature].Thumb N. Wien Klin Wochenschr Suppl. 1984; 156:44-8.
- Antiarthritic synergism of combined oral and parenteral chrysotherapy. I. Studies in adjuvant-induced arthritis in rats.[Inflammation. 1988]Antiarthritic synergism of combined oral and parenteral chrysotherapy. I. Studies in adjuvant-induced arthritis in rats.Finkelstein AE, Ladizesky M, Borinsky R, Kohn E, Ginsburg I. Inflammation. 1988 Aug; 12(4):373-82.
- Gold Preparations - LiverToxGold Preparations - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...