NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Glasdegib is an orally available small molecule inhibitor of the signaling molecule hedgehog which is used as an antineoplastic agent in the treatment of acute myeloid leukemia. Glasdegib is associated with a moderate rate of serum aminotransferase elevations during therapy and is suspected to cause rare instances of clinically apparent acute liver injury.
Background
Glasdegib (glas deg' ib) is a potent small molecule inhibitor of hedgehog, a signaling molecule that is frequently overexpressed in cancer cells including leukemia stem cells in patients with acute myeloid leukemia (AML). The hedgehog intracellular signaling cascade promotes cell growth and proliferation. Mutations in hedgehog are found in many types of cancer cells and can lead to unregulated cell growth. Glasdegib in combination with cytarabine with or without daunorubicin has been found to induce disease remissions and prolong overall survival in patients with AML and high-risk myelodysplastic syndromes who are unsuitable for conventional chemotherapy regimens. Glasdegib received accelerated approval for this indication in the United States in 2018 and is available in tablets of 40 mg under the brand name Daurismo. The recommended dose is 120 mg once daily, continued until progressive disease or intolerable toxicity occurs. Side effects are common and can include fatigue, myalgia, arthralgia, fever, diarrhea, nausea, abdominal pain, dizziness, headache, hypotension, cough and stomatitis. Uncommon, but potentially severe side effects include posterior reversible encephalopathy syndrome, febrile neutropenia and sepsis, QTc prolongation, pancreatitis and embryo-fetal toxicity.
Hepatotoxicity
Elevations in serum ALT levels are common during glasdegib therapy, occurring in 31% of patients and rising above 5 times the upper limit of the normal range in 11%. Glasdegib has had limited clinical use but has not been linked to instances of acute liver injury with symptoms or jaundice. Because of the limited clinical experience with the use of hedgehog inhibitors, their potential for causing liver injury is not well defined.
Likelihood score: E* (unproved but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The possible cause of the liver injury due to glasdegib is not known. Glasdegib is metabolized in the liver largely by the cytochrome P450 system (largely CYP 3A4) and is susceptible to drug-drug interactions with inhibitors or inducers of the microsomal enzyme system.
Outcome and Management
Glasdegib therapy has been associated with transient serum aminotransferase elevations during therapy, but has not been linked to instances of acute liver injury with jaundice or symptoms. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to temporary discontinuation, which should be permanent if laboratory values do not improve significantly or resolve within a few weeks or if symptoms or jaundice arise.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Glasdegib – Daurismo®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
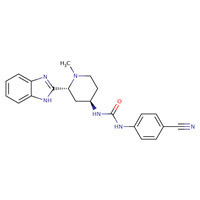
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Glasdegib | 1095173-27-5 | C21-H22-N6-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 20 January 2019
Abbreviation: AML, acute myelogenous leukemia.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of kinase inhibitors).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents published in 2013 before the availability of glasdegib and other hedgehog inhibitors).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway-targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions glasdegib- and comparator-treatment had similar rates of elevations in ALT [31% vs 28%], AST [36% vs 30%] and alkaline phosphatase [29% vs 30%], and no patient developed clinically apparent liver injury). - Savona MR, Pollyea DA, Stock W, Oehler VG, Schroeder MA, Lancet J, McCloskey J, et al. Phase Ib study of glasdegib, a hedgehog pathway inhibitor, in combination with standard chemotherapy in patients with AML or high-risk MDS. Clin Cancer Res 2018; 24: 2294-303. [PubMed: 29463550](Among 52 patients with AML or myelodysplastic syndromes treated with glasdegib [100 or 200 mg daily] combined with cytarabine or decitabine alone or cytarabine and daunorubicin, complete responses occurred in 16 [31%] and adverse event rates were common but considered manageable; no mention of ALT elevations or hepatotoxicity).
- Cortes JE, Douglas Smith B, Wang ES, Merchant A, Oehler VG, Arellano M, DeAngelo DJ, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: Phase 2 study results. Am J Hematol 2018; 93: 1301-10. [PMC free article: PMC6221102] [PubMed: 30074259](Among 69 patients with AML or myelodysplastic syndromes treated with glasdegib orally once daily with intravenous cytarabine and daunorubicin in six 28-day cycles, 47% had a complete remission, but all had adverse events that were often severe, including febrile neutropenia [64%], anemia [41%] and ALT elevations [30%], some being above 5 times ULN [5.7%]).
- Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, Montesinos P, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2019; 33: 379-89. [PMC free article: PMC6365492] [PubMed: 30555165](Among 132 patients with AML or high-risk myelodysplastic syndromes who were treated with intravenous cytarabine with or without oral glasdegib, median overall survival was greater with the combination [8.8 vs 4.9 months] while adverse events rates were similar, serious adverse events occurring in 78% vs 79% of patients and liver test abnormalities in 11% vs 10% all of which were transient, without symptoms or jaundice and not necessitating drug discontinuation).
- Hoy SM. Glasdegib: first global approval. Drugs 2019; 79: 207-13. [PubMed: 30666593](Review of the mechanism of action, history of development, pharmacology, efficacy and safety of glasdegib shortly after its initial approval as therapy of AML; mentions that liver enzyme elevations were frequent during glasdegib therapy, and they were not associated with jaundice or symptoms and did not require early drug discontinuation).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Gilteritinib.[LiverTox: Clinical and Researc...]Review Gilteritinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Midostaurin.[LiverTox: Clinical and Researc...]Review Midostaurin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ivosidenib.[LiverTox: Clinical and Researc...]Review Ivosidenib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Talazoparib.[LiverTox: Clinical and Researc...]Review Talazoparib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Glasdegib for the treatment of adult patients with newly diagnosed acute myeloid leukemia or high-grade myelodysplastic syndrome who are elderly or otherwise unfit for standard induction chemotherapy.[Drugs Today (Barc). 2019]Glasdegib for the treatment of adult patients with newly diagnosed acute myeloid leukemia or high-grade myelodysplastic syndrome who are elderly or otherwise unfit for standard induction chemotherapy.Goldsmith SR, Lovell AR, Schroeder MA. Drugs Today (Barc). 2019 Sep; 55(9):545-562.
- Glasdegib - LiverToxGlasdegib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...